Abstract
Purpose
New-onset olfactory and gustatory dysfunction (OGD) represents a well-acknowledged COVID-19 red flag. Nevertheless, its clinical, virological and serological features are still a matter of debate.
Materials and methods
For this cohort study, 170 consecutive subjects with new-onset OGD were consecutively recruited. Otolaryngological examination, OGD subjective grading, nasopharyngeal swabs (NS) for SARS-CoV-2 RNA detection and serum samples (SS) collection for SARS-CoV-2 IgG quantification were conducted at baseline and after one (T1), two (T2) and four weeks (T3).
Results
SARS-CoV-2 infection was confirmed in 79% of patients. Specifically, 43% of positive patients were detected only by SS analysis. The OGD was the only clinical complaint in 10% of cases. Concurrent sinonasal symptoms were reported by 45% of patients. Subjective improvement at T3 was reported by 97% of patients, with 40% recovering completely. Hormonal disorders and RNA detectability in NS were the only variables associated with OGD severity. Recovery rate was higher in case of seasonal influenza vaccination, lower in patients with systemic involvement and severe OGD. Not RNA levels nor IgG titers were correlated with recovery.
Conclusion
Clinical, virological and serological features of COVID-19 related OGD were monitored longitudinally, offering valuable hints for future research on the relationship between host characteristics and chemosensory dysfunctions.
Keywords: COVID-19, Smell, Taste, Immunoglobulins, RNA, Viral load
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), has been officially recognized as a pandemic by the World Health Organization (WHO), reaching at the time of writing (June 2021) more than 200 countries, with almost 178 million confirmed cases and more than 3 million deaths [1]. Besides nonspecific presenting symptoms, the olfactory and gustatory dysfunction (OGD) soon appeared to be one of the main features at onset, especially in paucisymptomatic cases and early phases of the disease [2], [3], [4]. The concomitance of OGD and viral infections is indeed a frequent finding [5], especially in otolaryngology, with OGD typically arising with nasal obstruction and discharge. Nevertheless, OGD in COVID-19 patients is weakly correlated with sinonasal symptoms. Moreover, it typically shows sudden and early onset, being often the only reported symptom [6], [7], [8], [9].
The prevalence of SARS-CoV-2 infection in new-onset OGD patients is well acknowledged, although it differs significantly between studies (74–94%) [10], [11], [12], [13]. Nevertheless, little is known about its connection with epidemiological variables and comorbidities. Furthermore, only few studies investigated the correlation between viral load on one side and the features of the chemosensitive dysfunction on the other, not taking into consideration serological parameters. Finally, to the best of our knowledge, no cross-sectional or prospective studies have been conducted to date employing both serology and molecular assays to better define the prevalence of SARS-CoV-2 infection in new-onset OGD.
The present study was therefore designed in order to longitudinally assess the prevalence of SARS-CoV-2 infection in new-onset OGD patients, basing on molecular and serological quantitative assays. In addition, OGD characteristics such as baseline severity, resolution rate and timing were investigated, correlating specific severity and resolution patterns to relevant clinical, virological and immunological features.
2. Materials and methods
The present observational cohort study was conducted at the Otolaryngology Department of Fondazione IRCCS Policlinico San Matteo (Pavia, Italy), after being approved by the internal review board (reference number: 20200041154). To ensure high quality presentation, the Strengthening the Reporting of Observational studies in Epidemiology guidelines were followed [14]. The study was conducted according to the World Medical Association Declaration of Helsinki.
2.1. Study population
Patients referred to our Department between March and May 2020 for new-onset OGD were consecutively recruited. Written informed consent was obtained from all participants. Inclusion criteria were as follows: age of 18 years or above; new-onset OGD. Exclusion criteria included: preexisting chronic OGD; chronic sinonasal pathologies; nasal decongestant abuse; substance abuse; neuropsychiatric disorders; major head and neck traumas; chemotherapy; radiation of the head and neck region. At the time of enrollment (T0), all participants underwent a baseline interview assessing general demographic and clinical variables (Table 1 ). A thorough ENT physical examination was conducted for all participants. Endoscopic examination was not performed, to prevent potential aerosolization of viral particles [15], [16].
Table 1.
Clinical and demographic features of the enrolled 170 patients presenting with new-onset olfactory and gustatory dysfunction.
| Variable (N = 170) | N (%) |
|---|---|
| Age (median; IQR) | 43; 30–51 |
| Gender | |
| Female | 108 (63.5) |
| Male | 62 (36.5) |
| Healthcare professional | 62 (36.5) |
| Influenza vaccination | 21 (12.4) |
| Smoking | |
| Never | 89 (52.4) |
| Former smoker | 33 (19.4) |
| Active smoker | 48 (28.2) |
| Allergies | 54 (31.8) |
| Comorbidities | 51 (30) |
| Cardiovascular | 16 (8.2) |
| Asthma | 4 (2.4) |
| Chronic obstructive pulmonary disease | 1 (0.6) |
| Endocrine disorders | 21 (12.4) |
| Autoimmune disorders | 11 (6.5) |
| Obstructive sleep apnea syndrome | 4 (2.4) |
| Other | 4 (2.4) |
IQR, interquartile range.
2.2. Molecular and serological testing for SARS-CoV-2
Nasopharyngeal swabs (NS) and serum samples (SS) were prospectively collected from all patients at T0 and after one (T1), two (T2) and four (T3) weeks. Detection and quantification of SARS-CoV-2 RNA were performed on samples collected from the rhinopharynx (FLOQSwabs™, Copan Italia, Brescia, Italy). RNA was extracted from 400 μL of Universal Transport Medium (UTM™) using the QIAsymphony® instrument with the QIAsymphony® DSP Virus/Pathogen Midi Kit (Complex 400 protocol), according to the manufacturer's instructions (QIAGEN, Qiagen, Hilden, Germany). Moreover, specific real-time RT-PCRs targeting RNA-dependent RNA polymerase and E genes were employed to detect the presence of SARS-CoV-2 according to the WHO guidelines1 and Corman's protocol [17]. Sequential SS were examined using chemiluminescent assay (Liason SARS-CoV-2 S1/S2 IgG, Diasorin, Saluggia, Italy) for quantitative characterization of SARS-CoV-2 anti-S1 and anti-S2 IgG antibodies. Results were given as AU/mL, and a cut-off of 15 AU/mL was set to define positive samples.
2.3. Olfactory and gustatory dysfunction (OGD)
At all times, a dedicated form was administered to investigate OGD features: type (“hyposmia/hypogeusia” versus “anosmia/ageusia”), date and type of onset (“sudden” versus “gradual”), course (“constant” versus “fluctuating”), general signs and symptoms (GSS), nasal symptoms (nasal obstruction, rhinorrhea). Since psychophysical tests were not practicable within COVID-19 emergency state, OGD subjective grading was conducted at all times through dedicated measures. First, a 100-millimiter Visual Analogue Scale (VAS), anchored at each end with verbal descriptors (“no impairment-0” and “extreme impairment-100”), was administered to investigate both gustatory (VAS-G) and olfactory (VAS—O) dysfunctions. The VAS has been already employed for OGD assessment. Moreover, significant correlations have been demonstrated between this scale and the Objective Odor Stick Tests (OOST) [18], [19], [20], [21]. Two additional patient-reported outcome (PRO) instruments were also administered: the Hyposmia Rating Scale (HRS) and the Chemosensory Complaint Score (CCS). The HRS was originally developed to grade olfactory dysfunctions in Parkinson's disease [22]. Each of the six items (scent of flowers; unburnt gas; garbage, sewage or other foul smelling materials; scent of perfume; smell of stuffiness or strong body odor; smell of home cooking) is rated on a five-point Likert scale from 1 (“always aware of the smell”) to 5 (“unfamiliar with or never smelt the smell”), with a total score ranging from 6 (no impairment) to 30 (worst impairment). Its clinical utility has been tested in several settings [22], [23], [24], demonstrating a strong correlation with the OOST. Taste impairments were graded instead using the CCS [25], originally developed to grade taste alterations in HIV patients and subsequently tested in chronic rhinosinusitis [26], which provides the best approximation of a patient-reported taste metrics. The CCS is an eight-item questionnaire investigating multiple features of taste dysfunctions: change in the sense of taste, change in the way food tastes, presence and quality of a bad taste, effect of medications on taste and changes in quality of four taste subgroups (salt, sweet, sour and bitter). Each of the items is given one point if abnormal. A ninth question deals with the overall severity of the dysfunction; it is given one point in case of a mild or moderate dysfunction or two points for a severe dysfunction. Thus, its total score ranges from 0 (no complaints) to 10 (severe complaints).
Given the context of the present pandemic, no validation studies of these PRO instruments were conducted. However, both the HRS and the CCS were preliminarily translated into Italian by two senior otolaryngologists and one bilingual translator to retain the meaning of the original items.
2.4. Statistical analysis
Qualitative variables were described as absolute frequencies and percentages. Quantitative variables were summarized in terms of median and interquartile range (IQR). Levels of the scales at T0 were compared according to epidemiological and clinical features using Mann-Whitney test or Kruskall-Wallis test (depending on whether the comparison was between two or more independent groups of patients). Scales trends during follow-up were evaluated by linear regression models. Standard errors were estimated with a clustered sandwich estimator in order to allow for intra-group correlation. Similarly, linear regression models with clustered sandwich standard errors were used to evaluate scales trends during follow-up as RNA copies/mL and IgG titers. Complete recovery free survival was calculated as the time between the enrollment and date of recovery or last follow-up. The crude effect (univariable analysis) of clinical and epidemiological features at T0 on complete recovery free survival was estimated by Cox Proportional Hazard models. Variables with a p-value lower than 0.2 in univariable analysis were candidate to enter in multivariable model. p values < 0.05 were considered statistically significant. All statistical analyses were conducted using the version 16 of the software Stata (StataCorp 2019; Stata Statistical Software: Release 16; College Station, TX: StataCorp LLC).
3. Results
3.1. Study population
Overall, 170 new-onset OGD patients were enrolled: 162 patients completed the follow-up (T3), while 3 and 2 patients were lost at T1 and T2, respectively. The median time from OGD onset to enrollment was 11 days (IQR 6–19). Table 1 summarizes demographic and clinical features of the participants. None of them had pathological findings at the ENT evaluation.
3.2. Molecular and serological testing for SARS-CoV-2
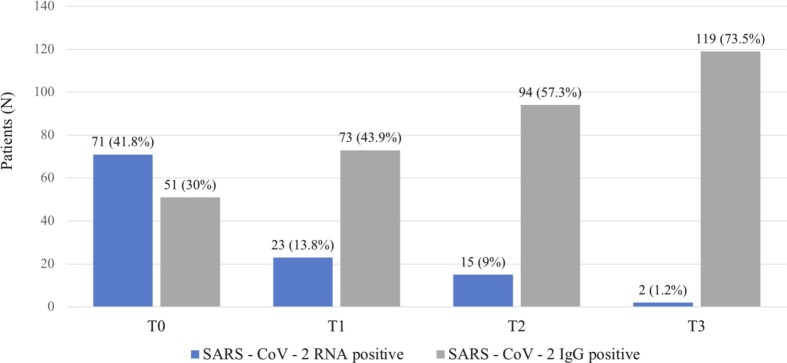
SARS-CoV-2 seroprevalence (SARS-CoV-2 IgG levels) and SARS-CoV-2 RNA copies in NS were evaluated over time (Fig. 1 ). Globally, SARS-CoV-2 infection was confirmed through NS and/or SS in 134 patients (79%; 95% CI: 72%–85%). SS analysis alone detected only 55 COVID-19 patients (43.3%).
Fig. 1.
SARS-CoV-2 seroprevalence (serum IgG positivity) and SARS-CoV-2 RNA detectability (nasopharyngeal swab positivity) at T0, T1, T2 and T3.
3.3. Olfactory and gustatory dysfunction (OGD)
Clinical features of SARS-CoV-2 patients are depicted in Table 2 . The OGD demonstrated sudden onset and constant intensity in most cases. Almost all patients (128; 96%) had a combined perceptual disorder. Seventy-four patients (55%) reported no additional sinonasal symptoms. When associated with nasal obstruction, the OGD occurred at the same time in 19 patients (44%), while it manifested before (median 2 days IQR 2–7) and after (median 5 days IQR 3–8) in 9 (21%) and 15 (35%) patients, respectively. Interestingly, an OGD with or without sinonasal symptoms was the only clinical complaint in 14 cases (11%). With regards to GSS, they occurred simultaneously with the OGD in 27 patients (25%), while they preceded (median 4 days IQR 2–7) or followed (median 5 days IQR 2–8) the OGD in 71 (65%) and in 11 (10%) subjects, respectively. No patients had severe COVID-19 symptoms requiring hospitalization during follow-up.
Table 2.
Clinical features of the olfactory and gustatory dysfunction in patients who tested positive for SARS-CoV-2 infection.
| Variable (N = 134) | N (%) |
|---|---|
| OGD characteristics | |
| Sudden onset | 123 (91.8) |
| Fluctuant | 15 (11.2) |
| Olfactory disorder only | 6 (4.5) |
| Taste disorder only | 0 (0.0) |
| Combined perceptual disorder | 128 (95.5) |
| Anosmia and ageusia | 100 (74.6) |
| Anosmia and hypogeusia | 15 (11.2) |
| Hyposmia and ageusia | 1 (0.7) |
| Hyposmia and hypogeusia | 12 (9.0) |
| Other sinonasal signs and symptoms | 60 (44.8) |
| Nasal obstruction | 43 (32.1) |
| Rhinorrhea | 44 (32.8) |
| General signs and symptoms | 120 (89.5) |
| Fever (>37.5 °C/99.5 °F) | 89 (66.4) |
| Headache | 73 (54.5) |
| Asthenia | 51 (38.1) |
| Cough | 38 (28.4) |
| Nausea | 23 (17.2) |
| Myalgia | 17 (12.7) |
| Diarrhea | 5 (3.7) |
| Dyspnea | 3 (2.2) |
| Pharyngodynia | 2 (1.5) |
| Vertigo | 1 (0.8) |
| None | 14 (10.5) |
OGD, olfactory and gustatory dysfunction.
OGD severity, although fluctuant in 15 patients (11%), showed an overall improving trend. In fact, both the baseline HRS score of 29 (IQR 27–30) and the baseline CCS score of 7 (IQR 5–8) significantly improved to 12 (IQR 6–22) and 0 (IQR 0–3) at T3, respectively (p < 0.001). Similarly, baseline VAS-O and VAS-G decreased from 9 (IQR 8–10) and 8 (IQR 5–10) to 2 (IQR 0–5) and 1 (IQR 0–4), respectively (p < 0.001). At the univariable analysis, endocrine disorders and positive NS were the only variables significantly correlated with OGD severity (Table 3 ). Precisely, in case of endocrine disorders or positive NS, patients reported higher subjective impairments. SARS-CoV-2 RNA levels in NS as well as IgG titers showed no significant correlation with OGD severity, not at baseline nor during follow-up (Table 4 ).
Table 3.
Association between epidemiological and clinical features and the olfactory and gustatory dysfunction severity at T0.
| VAS-O |
VAS-G |
HRS |
CCS |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Median (IQR) | p | Median (IQR) | p | Median (IQR) | p | Median (IQR) | p | |
| Sex | Female Male |
0.5 (0–3) 1 (0–2) |
0.769 | 2 (0–5) 2 (0–4.5) |
0.789 | 29 (26–30) 29 (27–30) |
0.987 | 7 (5–9) 6.5 (4.5–8) |
0.345 |
| Age (years) | <45 ≥45 |
0 (0–3) 1 (0–2) |
0.246 | 2 (0–5) 2 (1–4) |
0.654 | 30 (26–30) 29 (27–30) |
0.413 | 7 (5–9) 7 (4–8) |
0.492 |
| Smoking | No Ex Yes |
1 (0–2) 1 (0–3) 0 (0–2) |
0.673 | 2 (0–5) 2 (1–4) 1 (0–3) |
0.348 | 29 (26–30) 28 (27–30) 30 (25–30) |
0.939 | 7 (5–9) 7 (5–8) 7 (5–8) |
0.854 |
| Influenza vaccination | No Yes |
1 (0–2) 0 (0–2.5) |
0.475 | 2 (0–5) 2 (0.5–3) |
0.837 | 29 (26–30) 30 (27–30) |
0.379 | 7 (5–8) 7 (5–8.5) |
0.846 |
| Allergies | No Yes |
1 (0–3) 0 (0–2) |
0.234 | 2 (0–5) 2 (1–4) |
0.195 | 29 (26–30) 30 (27–30) |
0.394 | 7 (5–9) 7 (5–8) |
0.601 |
| Hormonal disorders | No Yes |
1 (0–3) 0 (0–1) |
0.134 | 2 (0–5) 0.5 (0–2) |
0.047* | 29 (26–30) 30 (28–30) |
0.045* | 7 (5–8) 9 (7–10) |
0.024* |
| General symptoms | No Yes |
0 (0–2) 1 (0–3) |
0.389 | 2 (0–4) 2 (0–5) |
0.442 | 29 (26–30) 29 (27–30) |
0.913 | 7 (4–9) 7 (5–8) |
0.708 |
| Nasal obstruction | No Yes |
0 (0–2) 1 (0–3) |
0.146 | 2 (0–4) 2 (0–5) |
0.428 | 29 (27–30) 28 (25–30) |
0.235 | 7 (5–9) 6.5 (5–8) |
0.464 |
| Sudden onset | No Yes |
2 (1–3) 0 (0–2) |
0.078 | 2 (1–5) 2 (0–5) |
0.310 | 28 (23−30) 29 (27–30) |
0.141 | 5 (3–7) 7 (5–9) |
0.053 |
| Concurrent pathologies | No Yes |
1 (0–2) 1 (0–2) |
0.920 | 2 (0–5) 2 (1–4) |
0.920 | 29 (27–30) 29 (27–30) |
0.959 | 7 (5–8) 7 (5–9) |
0.626 |
| SARS-CoV-2 RNA (NS) | NEG POS |
1 (0–3) 0 (0–2) |
0.001* | 2 (1–5) 2 (0–3) |
0.019* | 28 (25–30) 30 (28–30) |
0.001* | 7 (4–8) 7 (5–9) |
0.189 |
VAS-O, Visual Analogue Scale – Olfactory; VAS-G, Visual Analogue Scale – Gustatory; HRS, Hyposmia Rating Scale; CCS, Chemosensory Complaint Score; IQR, interquartile range; NS, nasal swab; NEG, negative; POS, positive; * p < 0.05
Table 4.
Correlations between log10 SARS-CoV-2 RNA copies/mL, SARS-CoV-2 IgG titers and OGD severity at baseline and during follow-up.
| Log10 RNA copies/mL |
IgG titers |
|||
|---|---|---|---|---|
| T0 | Follow-up | T0 | Follow-up | |
| VAS-O | R = −0.05, p = 0.690 | Coef = 0.11, p = 0.841 | R = 0.13, p = 0.337 | Coef = −0.02, p = 0.943 |
| VAS-G | R = 0.05, p = 0.657 | Coef = 0.76, p = 0.379 | R = 0.10, p = 0.435 | Coef = 0.08, p = 0.771 |
| HRS | R = 0.07, p = 0.558 | Coef = −0.9, p = 0358 | R = −0.30, p = 0.051 | Coef = −0.11, p = 0.866 |
| CCS | R = −0.01, p = 0.974 | Coef = −0.5, p = 0.569 | R = −0.14, p = 0.285 | Coef = −0.04, p = 0.882 |
VAS-O, Visual Analogue Scale – Olfactory; VAS-G, Visual Analogue Scale – Gustatory; HRS, Hyposmia Rating Scale; CCS, Chemosensory Complaint Score.
As far as recovery is concerned, 130 patients (97%) reported an improvement of the OGD at T3, but only 53 (40%) recovered completely within 23 days from onset (IQR 18–32). Specifically, higher severity at onset and GSS were associated with a lower probability of complete recovery within T3 (Table 5 ). A mild association between influenza vaccination and complete OGD recovery was also demonstrated through the multivariable analysis (Table 6 ). No other relevant associations have been highlighted. SARS-CoV-2 RNA detectability in NS and SARS-CoV-2 IgG positivity in SS were not associated with OGD recovery rate (p = 0.523 and p = 0.214, respectively).
Table 5.
Univariable analysis investigating the influence of epidemiological and clinical variables on the olfactory and gustatory dysfunction (OGD) resolution at T3.
| HR | 95%CI | p | |
|---|---|---|---|
| Age (years) | 0.98 | 0.96–1.01 | 0.147 |
| Sex (male vs female) | 0.8 | 0.4–1.4 | 0.454 |
| Seasonal influenza vaccination (yes vs no) | 2.0 | 1.0–4.0 | 0.070* |
| Smoking | |||
| Ex vs no | 1.4 | 0.7–2.9 | 0.338 |
| Yes vs no | 1.5 | 0.8–2.9 | 0.235 |
| General sign and symptoms (yes vs no) | 0.5 | 0.3–0.9 | 0.023* |
| Nasal obstruction (yes vs no) | 1.4 | 0.8–2.5 | 0.238 |
| Comorbidities (yes vs no) | 0.8 | 0.4–1.6 | 0.547 |
| Endocrine disorders (yes vs no) | 0.9 | 0.4–2.3 | 0.866 |
| Allergies (yes vs no) | 1.2 | 0.6–2.1 | 0.632 |
| Rhinorrhea (yes vs no) | 0.8 | 0.4–1.5 | 0.463 |
| SARS-CoV-2 IgG titer (AU/mL) | 0.9 | 0.6–1.6 | 0.801 |
| Log10 RNA (copies/mL) | 0.8 | 0.3–2.0 | 0.644 |
| VAS-O | 1.1 | 1.0–1.3 | 0.083 |
| VAS-G | 1.1 | 1.0–1.2 | 0.005* |
| HRS | 0.9 | 0.9–1.0 | 0.034* |
| CCS | 0.9 | 0.8–1.0 | 0.003* |
HR, Hazard Ratio; CI, Confidence Interval; VAS—O, Visual Analogue Scale – Olfactory; VAS-G, Visual Analogue Scale – Gustatory; HRS, Hyposmia Rating Scale; CCS, Chemosensory Complaint Score; * p < 0.05.
Table 6.
Multivariable analysis of epidemiological and clinical variables related to OGD resolution at the end of the follow-up period (T3).
| Model 1 (HRS) |
Model 2 (CCS) |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age (years) | 0.99 | 0.97–1.01 | 0.361 | 0.99 | 0.96–1.01 | 0.232 |
| Seasonal influenza vaccination (yes vs no) | 2.33 | 1.10–4.95 | 0.027 | 2.42 | 1.14–5.14 | 0.021 |
| General sign and symptoms (yes vs no) | 0.54 | 0.29–1.01 | 0.053 | 0.54 | 0.29–1.00 | 0.051 |
| HRS | 0.93 | 0.88–0.98 | 0.009 | – | – | – |
| CCS | – | – | – | 0.86 | 0.79–0.94 | 0.001 |
HR, Hazard Ratio; CI, Confidence Interval; HRS, Hyposmia Rating Scale; CCS, Chemosensory Complaint Score.
4. Discussion
This study aimed to investigate the clinical, virological and immunological features of the COVID-19-related OGD over time. Several studies demonstrated that the OGD is a strong clinical marker of COVID-19, with a positive predictive value of 61% [7], even though its relevant specificity (93–99%) is not matched by an equally high sensitivity (23–43%) [6]. In our investigation, the OGD confirmed its clinical relevance, being the only reported complaint in 10.5% of positive patients. The chemosensory impairment demonstrated fundamental importance for diagnosis even in case of systemic disease, since in 10.1% of patients the OGD anticipated GSS by 5 days (IQR 2–8).
The overall prevalence of SARS-CoV-2 infection, based on NS and SS analyses combined, was 79%. Most studies investigating SARS-CoV-2 prevalence in OGD patients relied on NS results one, like the reports from Lechien (88%) [10], Salmon-Ceron (94%) [11] and Hopkins (74%) [27]. Only two studies so far employs SS testing, specifically a preliminary report from our study group (75%) [9] and a cross-sectional study employing solely qualitative point-of-care serological kits (77.6%) [28]. To the best of our knowledge, this is the first study assessing SARS-CoV-2 prevalence relying on both NS and SS within a controlled clinical setting. Noteworthily, our results highlighted the cruciality of serological testing for COVID-19 diagnosis in OGD patients. In fact, more than 40% of COVID-19 cases were detected only by means of serological essays, implying that NS or SS alone could have underestimated SARS-CoV-2 prevalence. This may be primarily related to the interval between symptoms' onset and laboratory confirmation. Indeed, as we previously observed in a smaller cohort [9], the great majority of patients developed SARS-CoV-2 IgG three weeks after symptoms' onset, while RNA detectability in NS gradually decreased over time (Fig. 1).
As far as clinical features are concerned, literature reveals how COVID-19-related OGD usually presents as a combined disorder, affecting both smell and taste in most cases [6], [7], [29], [30], [31]. Our results align with published data, with 96% of patients reporting both impairments. In fact, even though retro-nasal olfaction may contribute to flavors perception, emerging evidence depicts direct viral involvement as the primary mechanism underlying COVID-19 gustatory dysfunction [5]. Interestingly, the expression of Angiotensin-Converting Enzyme-2 (ACE-2) – the principal cell receptor for SARS-CoV-2 – has been recently identified in taste organs of murine models [32], [33].
With regards to baseline OGD severity, only endocrine disorders, as found by Lechien et al. [38], and RNA detectability in NS were significantly associated with higher impairments. The influence of metabolic disorders on smell and taste is well-acknowledged [34], [35], [36], since the olfactory and the endocrine system are intimately linked. Axonal projections to and from the olfactory bulb allow a crosstalk between the olfactory system and the hypothalamus. Moreover, the olfactory mucosa and bulb cells express receptors and peptides involved in metabolic homeostasis [37].
Despite the overall improving trend, only 40% of patients recovered completely from the OGD at T3. Resolution rate appear to be variable in literature, ranging from 13% [39] to 86% [40], mainly depending on follow-up duration and OGD evaluation methodology (quantitative vs qualitative) [41], [42]. In our sample, severity at onset was the most relevant variable influencing complete resolution. This evidence is confirmed by other studies investigating the evolution of chemosensory impairments in COVID-19 [43], [44], [45], [46], [47]. To the best of our knowledge, the association between OGD and GSS has never been deeply investigated to date. In our study, GSS were associated with lower chances of complete recovery. It might be speculated that systemic involvement could reflect a more severe SARS-CoV-2 infection and, therefore, reduced abilities of the body to cope with the infection. Interestingly, Lovato et al. [48], in their preliminary report on 121 COVID-19 patients, found out that among GSS, the absence of fever was significantly associated with persistent OTD. Authors speculate that, since fever is associated with severe COVID-19, patients with OGD without fever would experience a mild-moderate COVID-19. However, to date no final inferences can be drawn in this matter, and further studies are needed to properly test this hypothesis.
Impressively, a significant association between influenza vaccination and complete recovery at T3 was also verified. Similar trends were also noted before the present pandemic by Flanagan, who highlighted how influenza vaccination rates were significantly lower among OGD patients [49]. Furthermore, this association was also explored on murine models, in which intranasal immunization demonstrated attenuation properties on both viral localization and inflammation of the olfactory bulb [50]. These findings and our results offer interesting hints for future clinical and experimental research, which should be aimed at better understanding the connection between acquired immunity and chemosensory dysfunctions.
Several virological and immunological features of COVID-19 OGD appear noteworthy. First, the only significant virological correlation was found between RNA detectability in NS on one side and OGD baseline severity on the other. Surprisingly, while the presence of SARS-CoV-2 in the nasopharynx was associated with higher perceptual impairments, RNA copies/mL never showed significant correlations with OGD severity. These findings suggest that active SARS-CoV-2 infections might be the only prerequisite for the development of perceptual impairments, not necessarily requiring higher viral loads to reach clinical relevance. The relationship between the viral load and the chemosensory dysfunction is still a matter of debate. Jain et al., comparing the cycle threshold (CT) value on PCR assay in COVID-19 patients with and without OGD, found out that patients with olfactory dysfunction had higher viral load than those without perceptual impairment [51]. However, Vaira et al. and Cho et al. did not find a significant correlation between the CT value and the olfactory function, suggesting that the OGD severity might be related to individual susceptibility rather than viral load [52], [53]. Further studies investigating the correlation of viral load and the chemosensory dysfunction COVID-19 related are needed.
With regards to immunological data, no significant associations were found between IgG titers and overall OGD severity or trend. These results appear to be quite unexpected, since higher IgG titers should correspond to more effective responses to the infection and, theoretically, less severe and less lasting symptoms. Nevertheless, also this serological evidence needs further investigation.
Sinonasal symptoms other than the chemosensory impairment itself (i.e., nasal obstruction and nasal discharge) affected less than half of positive OGD patients. However, Naeini and colleagues demonstrated through computed tomography of paranasal sinuses that there were no significant pathological changes in the olfactory clefts and sinonasal mucosa in the 49 anosmic COVID-19 patients analysed [54].
Furthermore, no significant differences in terms of OGD baseline severity, course and resolution rate were found between patients with and without nasal complaints. Notoriously, sinonasal symptoms alone may lead to olfactory dysfunctions, since nasal inflammation and mucosal swelling can prevent olfactory molecules from reaching the olfactory clefts. The persistence of olfactory impairments without major nasal complaints and after the acute phase of COVID-19 strengthens the hypothesis of a direct damage of both the olfactory epithelium and central olfactory pathways. SARS-CoV-2 is a neurotropic virus, able to spread from the peripheral olfactory system to the central nervous system [55], [56], [57]. Several reports have demonstrated viral spreading towards the olfactory bulb through the olfactory neuroepithelium [10], [58], [59]. ACE-2 and TMPRSS2, required for SARS-CoV-2 entry in host cells, were detected in sustentacular and olfactory stem cells in human specimens [60], [61]. Central nervous system involvement by SARS-CoV-2 has been proven even radiologically, with MRI evidence of olfactory bulb abnormalities in anosmic COVID-19 patients [62], [63]. Moreover, SARS-CoV-2 particles, diffuse infiltration of CD163-positive macrophages and cytotoxic T lymphocytes have been identified in the olfactory bulbs of patients with severe COVID-19 [64]. Moreover, there is emerging evidence regarding the role of SARS-CoV-2 in determining olfactory impairment through blocking the rapid turnover of the olfactory receptors. In fact, according to recent studies, SARS-CoV-2 might attack the nasal serous gland inhibiting the production of the growth factors necessary for stem cells activation and transformation in olfactory receptors [65]. In conclusion, the contribution of different potential pathogeneses to the olfactory impairment of COVID-19 deserves further studies.
This study has several limitations. Firstly, due to the COVID-19 emergency state, the OGD was not evaluated with psychophysical tests or electrophysiological studies, since they were not practicable. These instruments could be potentially employed to screen residual long-lasting OGD after the resolution of the emergency state. Secondly, although a thirty-day follow-up is significantly longer than the average follow-up of previous reports, longer monitoring could have better contributed in evaluating the rate of persistent OGD. Lastly, in reason of the current emergency, OGD etiologies in SARS-CoV-2 negative patients were not investigated.
5. Conclusion
The present longitudinal study depicts the clinical course of COVID-19-related OGD with regards to its virological and immunological features. Specifically, the combination of SS and NS appeared to be crucial in order to identify a higher rate of positive COVID-19 patients suffering from OGD. A higher OGD severity was reported in case of RNA detectability in NS and concurrent endocrine disorders. Recovery rates were lower in case of higher severity at onset, as well as in case of systemic symptoms. Contrariwise, a higher recovery rate was highlighted for subjects who underwent influenza vaccination, offering hints for future research investigating the relationship between acquired immunity and chemosensory dysfunctions.
Previous presentations
The manuscript was not previously presented.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
EM: patient enrollment, patient examination and testing, questionnaire administration, literature revision, drafting of the manuscript; AC: patient enrollment, patient examination and testing, questionnaire administration, literature revision; CR: literature revision, drafting of the manuscript; IC: analysis of blood samples and nasal swabs specimens; VZ, ES: patient enrollment; VVF, CK: data analysis and interpretation; FB, MB: study design, critical revision of the manuscript.
Declaration of competing interest
None.
Acknowledgements
We would like to thank all participants enrolled in the present study. This paper is dedicated to all healthcare workers fighting against COVID-19.
References
- 1.World Health Organization Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 2.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giacomelli A., Pezzati L., Conti F., et al. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis. 2020;71(15):889–890. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mercante G., Ferreli F., De Virgilio A., et al. Prevalence of taste and smell dysfunction in coronavirus disease 2019. JAMA Otolaryngol Head Neck Surg. 2020;146(8):1–6. doi: 10.1001/jamaoto.2020.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hummel T., Whitcroft K.L., Andrews P., et al. Position paper on olfactory dysfunction. Rhinology. 2017;54(26):1–30. doi: 10.4193/Rhino16.248. [DOI] [PubMed] [Google Scholar]
- 6.Agyeman A.A., Chin K.L., Landersdorfer C.B., Liew D., Ofori-Asenso R. Smell and taste dysfunction in patients with COVID-19: a systematic review and meta-analysis. Mayo Clin Proc. 2020;95(8):1621–1631. doi: 10.1016/j.mayocp.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rocke J., Hopkins C., Philpott C., Kumar N. Is loss of sense of smell a diagnostic marker in COVID-19: a systematic review and meta-analysis [published online ahead of print, 2020 Aug 1] Clin Otolaryngol. 2020 doi: 10.1111/coa.13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Oto-Rhino-Laryngology. 2020;277(8):2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benazzo M., Cassaniti I., Maiorano E., et al. Sars-cov-2 virologic and immunologic correlates in patients with olfactory and taste disorders. Microorganisms. 2020;8(7):1052. doi: 10.3390/microorganisms8071052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lechien J.R., Cabaraux P., Chiesa-Estomba C.M., et al. Psychophysical olfactory tests and detection of COVID-19 in patients with sudden onset olfactory dysfunction: a prospective study. Ear Nose Throat J. 2020;99(9):579–583. doi: 10.1177/0145561320929169. [DOI] [PubMed] [Google Scholar]
- 11.Salmon Ceron D., Bartier S., Hautefort C., et al. Self-reported loss of smell without nasal obstruction to identify COVID-19. The multicenter Coranosmia cohort study. J Infect. 2020;81(4):614–620. doi: 10.1016/j.jinf.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seden N., Yigit E., Yigit Ö., Kaygisiz I. Objective evaluation of odor loss in COVID-19 and other suspected cases. Am J Otolaryngol. 2021 Jan-Feb;42(1) doi: 10.1016/j.amjoto.2020.102761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng X., Deng Y., Dai Z., Meng Z. COVID-19 and anosmia: a review based on up-to-date knowledge. Am J Otolaryngol. 2020 Sep-Oct;41(5) doi: 10.1016/j.amjoto.2020.102581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Kay J.K., Parsel S.M., Marsh J.J., McWhorter A.J., Friedlander P.L. Risk of SARS-CoV-2 transmission during flexible laryngoscopy: a systematic review. JAMA Otolaryngol Head Neck Surg. 2020;146(9):851–856. doi: 10.1001/jamaoto.2020.1973. [DOI] [PubMed] [Google Scholar]
- 16.Zhao C., Viana A., Jr., Wang Y., Wei H.Q., Yan A.H., Capasso R. Otolaryngology during COVID-19: preventive care and precautionary measures. Am J Otolaryngol. 2020 Jul-Aug;41(4) doi: 10.1016/j.amjoto.2020.102508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corman V.M., Landt O., Kaiser M., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crichton N. Visual analogue scale (VAS) J Clin Nurs. 2001;10:697–706. [Google Scholar]
- 19.Ellegård E.K., Goldsmith D., Hay K.D., Morton R.P. Studies on the relationship between electrogustometry and sour taste perception. Auris Nasus Larynx. 2007;34(4):477–480. doi: 10.1016/j.anl.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto Y., Fukazawa K., Fujii M., et al. Usefulness of the odor stick identification test for Japanese patients with olfactory dysfunction. Chem Senses. 2004;29(7):565–571. doi: 10.1093/chemse/bjh061. [DOI] [PubMed] [Google Scholar]
- 21.Haxel B.R., Bertz-Duffy S., Fruth K., Letzel S., Mann W.J., Muttray A. Comparison of subjective olfaction ratings in patients with and without olfactory disorders. J Laryngol Otol. 2012;126(7):692–697. doi: 10.1017/S002221511200076X. [DOI] [PubMed] [Google Scholar]
- 22.Millar Vernetti P., Perez Lloret S., Rossi M., Cerquetti D., Merello M. Validation of a new scale to assess olfactory dysfunction in patients with Parkinson's disease. Park Relat Disord. 2012;18(4):358–361. doi: 10.1016/j.parkreldis.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Galletti B., Santoro R.R., Mannella V.K., et al. Olfactory event-related potentials: a new approach for the evaluation of olfaction in nasopharyngeal carcinoma patients treated with chemo-radiotherapy. J Laryngol Otol. 2016;130(5):453–461. doi: 10.1017/S0022215116000761. [DOI] [PubMed] [Google Scholar]
- 24.Yu Q., Guo P., Li D., et al. Olfactory dysfunction and its relationship with clinical symptoms of alzheimer disease. Aging Dis. 2018;9(6):1084–1095. doi: 10.14336/AD.2018.0819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heald A.E., Pieper C.F., Schiffman S.S. Taste and smell complaints in HIV-infected patients. AIDS. 1998;12(13):1667–1674. doi: 10.1097/00002030-199813000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Othieno F., Schlosser R.J., Rowan N.R., et al. Taste impairment in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2018;8(7):783–789. doi: 10.1002/alr.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makaronidis J., Mok J., Balogun N., Magee C.G., Omar R.Z., Carnemolla A., Batterham R.L. Seroprevalence of SARS-CoV-2 antibodies in people with an acute loss in their sense of smell and/or taste in a community-based population in London, UK: an observational cohort study. PLoS Med. 2020 Oct 1;17(10) doi: 10.1371/journal.pmed.1003358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hopkins C., Surda P., Nirmal Kumar B. Presentation of new onset anosmia during the covid-19 pandemic. Rhinology. 2020;58(3):295–298. doi: 10.4193/Rhin20.116. [DOI] [PubMed] [Google Scholar]
- 29.Husain Q., Kokinakos K., Kuo Y.H., Zaidi F., Houston S., Shargorodsky J. Characteristics of COVID-19 smell and taste dysfunction in hospitalized patients. Am J Otolaryngol. 2021 Apr 19;42(6) doi: 10.1016/j.amjoto.2021.103068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raad R.A., Ganti A., Goshtasbi K., Lehrich B.M., Papagiannopoulos P., LoSavio P., Mahdavinia M., Kuan E.C., Batra P.S., Tajudeen B.A. Temporal patterns of nasal symptoms in patients with mild severity SARS-CoV-2 infection. Am J Otolaryngol. 2021 Apr 24;42(6) doi: 10.1016/j.amjoto.2021.103076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fantozzi P.J., Pampena E., Di Vanna D., Pellegrino E., Corbi D., Mammucari S., Alessi F., Pampena R., Bertazzoni G., Minisola S., Mastroianni C.M., Polimeni A., Romeo U., Villa A. Xerostomia, gustatory and olfactory dysfunctions in patients with COVID-19. Am J Otolaryngol. 2020 Nov-Dec;41(6) doi: 10.1016/j.amjoto.2020.102721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shigemura N., Takai S., Hirose F., Yoshida R., Sanematsu K., Ninomiya Y. Expression of renin-angiotensin system components in the taste organ of mice. Nutrients. 2019;11(9):2251. doi: 10.3390/nu11092251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swidzinski T., Linkowska-Swidzinska K., Czerniejewska-Wolska H., et al. Hypothyroidism affects olfactory evoked potentials. Biomed Res Int. 2016;2016:9583495. doi: 10.1155/2016/9583495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deniz F., Ay S.A., Salihoglu M., et al. Thyroid hormone replacement therapy improves olfaction and taste sensitivity in primary hypothyroid patients: a prospective randomised clinical trial. Exp Clin Endocrinol Diabetes. 2016;124(9):562–567. doi: 10.1055/s-0042-108446. [DOI] [PubMed] [Google Scholar]
- 36.McConnell R.J., Menendez C.E., Smith F.R., Henkin R.I., Rivlin R.S. Defects of taste and smell in patients with hypothyroidism. Am J Med. 1975;59(3):354–364. doi: 10.1016/0002-9343(75)90394-0. [DOI] [PubMed] [Google Scholar]
- 37.Palouzier-Paulignan B., Lacroix M.-C., Aimé P., et al. Olfaction under metabolic influences. Chem Senses. 2012;37:769–797. doi: 10.1093/chemse/bjs059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lechien J.R., Chiesa-Estomba C.M., Vaira L.A., De Riu G., Cammaroto G., Chekkoury-Idrissi Y., Circiu M., Distinguin L., Journe F., de Terwangne C., Machayekhi S., Barillari M.R., Calvo-Henriquez C., Hans S., Saussez S. Epidemiological, otolaryngological, olfactory and gustatory outcomes according to the severity of COVID-19: a study of 2579 patients. Eur Arch Otorhinolaryngol. 2021 Jan;16:1–9. doi: 10.1007/s00405-020-06548-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaye R., Chang C.W.D., Kazahaya K., Brereton J., Denneny J.C. COVID-19 anosmia reporting tool: initial findings. Otolaryngol Head Neck Surg. 2020;163(1):132–134. doi: 10.1177/0194599820922992. [DOI] [PubMed] [Google Scholar]
- 40.D'Ascanio L., Pandolfini M., Cingolani C., Latini G., Gradoni P., Capalbo M., Frausini G., Maranzano M., Brenner M.J., Di Stadio A. Olfactory dysfunction in COVID-19 patients: prevalence and prognosis for recovering sense of smell. Otolaryngol Head Neck Surg. 2021 Jan;164(1):82–86. doi: 10.1177/0194599820943530. [DOI] [PubMed] [Google Scholar]
- 41.Fuccillo E., Saibene A.M., Canevini M.P., Felisati G. Olfactory disorders in coronavirus disease 2019 patients: a systematic literature review. J Laryngol Otol. 2020 Sep;15:1–10. doi: 10.1017/S0022215120002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santos R.E.A., da Silva M.G., do Monte Silva MCB Barbosa D.A.M., Gomes ALDV Onset and duration of symptoms of loss of smell/taste in patients with COVID-19: a systematic review. Am J Otolaryngol. 2021 Mar-Apr;42(2) doi: 10.1016/j.amjoto.2020.102889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boscolo-Rizzo P., Borsetto D., Fabbris C., et al. Evolution of altered sense of smell or taste in patients with mildly symptomatic COVID-19. JAMA Otolaryngol Head Neck Surg. 2020;146(8):729–732. doi: 10.1001/jamaoto.2020.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Speth M.M., Singer-Cornelius T., Oberle M., Gengler I., Brockmeier S.J., Sedaghat A.R. Time scale for resolution of olfactory dysfunction in COVID-19. Rhinol J. 2020;58(4):404–405. doi: 10.4193/Rhin20.227. [DOI] [PubMed] [Google Scholar]
- 45.Paderno A., Mattavelli D., Rampinelli V., et al. Olfactory and gustatory outcomes in COVID-19: a prospective evaluation in nonhospitalized subjects. Otolaryngol Head Neck Surg. 2020;163(6):1144–1149. doi: 10.1177/0194599820939538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amer M.A., Elsherif H.S., Abdel-Hamid A.S., Elzayat S. Early recovery patterns of olfactory disorders in COVID-19 patients; a clinical cohort study. Am J Otolaryngol. 2020 Nov-Dec;41(6) doi: 10.1016/j.amjoto.2020.102725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Biadsee A., Dagan O., Ormianer Z., Kassem F., Masarwa S., Biadsee A. Eight-month follow-up of olfactory and gustatory dysfunctions in recovered COVID-19 patients. Am J Otolaryngol. 2021 Jul-Aug;42(4) doi: 10.1016/j.amjoto.2021.103065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lovato A., Galletti C., Galletti B., de Filippis C. Clinical characteristics associated with persistent olfactory and taste alterations in COVID-19: a preliminary report on 121 patients. Am J Otolaryngol. 2020 Sep-Oct;41(5) doi: 10.1016/j.amjoto.2020.102548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flanagan C.E., Wise S.K., DelGaudio J.M., Patel Z.M. Association of decreased rate of influenza vaccination with increased subjective olfactory dysfunction. JAMA Otolaryngol Head Neck Surg. 2015;141(3):225–228. doi: 10.1001/jamaoto.2014.3399. [DOI] [PubMed] [Google Scholar]
- 50.Reden J., Mueller A., Mueller C., et al. Recovery of olfactory function following closed head injury or infections of the upper respiratory tract. Arch Otolaryngol Head Neck Surg. 2006;132(3):265–269. doi: 10.1001/archotol.132.3.265. [DOI] [PubMed] [Google Scholar]
- 51.Vaira L.A., Lechien J.R., De Riu G., Saussez S. Chemosensory dysfunction in COVID-19: is there really a correlation with viral load? Am J Otolaryngol. 2021 Jul-Aug;42(4) doi: 10.1016/j.amjoto.2021.103037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cho R.H., To Z.W.H., Yeung Z.W.C., Tso E.Y.K., Fung K.S.C., Chau S.K.Y. COVID-19 viral load in the severity of and recovery from olfactory and gustatory dysfunction. Laryngoscope. 2020;130:2680–2685. doi: 10.1002/lary.29056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jain A., Pandey A.K., Kaur J., Kumar L., Singh M., Das S., Purohit S. Is there a correlation between viral load and olfactory & taste dysfunction in COVID-19 patients? Am J Otolaryngol. 2021 May-Jun;42(3) doi: 10.1016/j.amjoto.2021.102911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naeini A.S., Karimi-Galougahi M., Raad N., Ghorbani J., Taraghi A., Haseli S., Mehrparvar G., Bakhshayeshkaram M. Paranasal sinuses computed tomography findings in anosmia of COVID-19. Am J Otolaryngol. 2020 Nov-Dec;41(6) doi: 10.1016/j.amjoto.2020.102636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tassorelli C., Mojoli F., Baldanti F., Bruno R., Benazzo M. COVID-19: what if the brain had a role in causing the deaths? Eur J Neurol. 2020;27(9):e41–e42. doi: 10.1111/ene.14275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gorzkowski V., Bevilacqua S., Charmillon A., et al. Evolution of olfactory disorders in COVID-19 patients. Laryngoscope. 2020;130(11):2667–2673. doi: 10.1002/lary.28957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Freni F., Meduri A., Gazia F., Nicastro V., Galletti C., Aragona P., Galletti C., Galletti B., Galletti F. Symptomatology in head and neck district in coronavirus disease (COVID-19): a possible neuroinvasive action of SARS-CoV-2. Am J Otolaryngol. 2020 Sep-Oct;41(5) doi: 10.1016/j.amjoto.2020.102612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zubair A.S., McAlpine L.S., Gardin T., Farhadian S., Kuruvilla D.E., Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77(8):1018–1027. doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Renaud M., Leon A., Trau G., et al. Acute smell and taste loss in outpatients: all infected with SARS-CoV-2? Rhinology. 2020;58(4):406–409. doi: 10.4193/Rhin20.199. [DOI] [PubMed] [Google Scholar]
- 60.Bilinska K., Jakubowska P., Von Bartheld C.S., Butowt R. Expression of the SARS-CoV-2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: identification of cell types and trends with age. ACS Chem Nerosci. 2020;11(11):1555–1562. doi: 10.1021/acschemneuro.0c00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brann D.H., Tsukahara T., Weinreb C., et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv. 2020;6(31) doi: 10.1126/sciadv.abc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Politi L.S., Salsano E., Grimaldi M. Magnetic resonance imaging alteration of the brain in a patient with coronavirus disease 2019 (COVID-19) and anosmia. JAMA Neurol. 2020;77(8):1028–1029. doi: 10.1001/jamaneurol.2020.2125. [DOI] [PubMed] [Google Scholar]
- 63.Chetrit A., Lechien J.R., Ammar A., et al. Magnetic resonance imaging of COVID-19 anosmic patients reveals abnormalities of the olfactory bulb: preliminary prospective study. J Infect. 2020;81(5):816–846. doi: 10.1016/j.jinf.2020.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morbini P., Benazzo M., Verga L., et al. Ultrastructural evidence of direct viral damage to the olfactory complex in patients testing positive for SARS-CoV-2. JAMA Otolaryngol Head Neck Surg. 2020;146(10):972–973. doi: 10.1001/jamaoto.2020.2366. [DOI] [PubMed] [Google Scholar]
- 65.Henkin R.I. How does Covid-19 infection affect smell? Am J Otolaryngol. 2021 May-Jun;42(3) doi: 10.1016/j.amjoto.2021.102912. Epub 2021 Jan 27. [DOI] [PMC free article] [PubMed] [Google Scholar]