Abstract
Exposure to arsenic (As) is a major public health concern globally. Inorganic As (InAs) undergoes hepatic methylation to form monomethyl (MMAs)- and dimethyl (DMAs)-arsenical species, facilitating urinary As elimination. MMAsIII is considerably more toxic than either InAsIII or DMAsV, and a higher proportion of MMAs in urine has been associated with risk for a wide range of adverse health outcomes. Efficiency of As methylation differs substantially between species, between individuals, and across populations. One-carbon metabolism (OCM) is a biochemical pathway that provides methyl groups for the methylation of As, and is influenced by folate and other micronutrients, such as vitamin B12, choline, betaine and creatine. A growing body of evidence has demonstrated that OCM-related micronutrients play a critical role in As methylation. This review will summarize observational epidemiological studies, interventions, and relevant experimental evidence examining the role that OCM-related micronutrients have on As methylation, toxicity of As, and risk for associated adverse health-related outcomes. There is fairly robust evidence supporting the impact of folate on As methylation, and some evidence from case-control studies indicating that folate nutritional status influences risk for As-induced skin lesions and bladder cancer. However, the potential for folate to be protective for other As-related health outcomes, and the potential beneficial effects of other OCM-related micronutrients on As methylation and risk for health outcomes are less well studied and warrant additional research.
Keywords: folate, one-carbon metabolism, arsenic, arsenic methylation, nutrition
1. Introduction
Arsenic (As) exposure through drinking water and food is a worldwide public health concern. In a process dependent on one-carbon metabolism (OCM), ingested inorganic As (InAs) is methylated to form monomethyl (MMAs) and dimethyl (DMAs) arsenicals. Complete methylation of InAs to DMAs facilitates urinary As elimination and reduces As-related toxicity. OCM is a biochemical pathway that is influenced by nutrients including folate, vitamin B12, choline, betaine and creatine. This article is an update of our review published in 2018,1 and provides an overview of As exposure, methylation, toxicity, and risk for As-related health outcomes. We then summarize the evidence that nutritional status and interventions that influence OCM nutrient levels can increase As methylation capacity and potentially reduce the risk of As-related toxicity. We will primarily focus on human studies of folate. These findings have important implications for many countries with the highest levels of As exposure, as these regions also tend to have a high prevalence of folate deficiency and could benefit from major public health interventions such as food folate fortification programs currently mandated in more than 80 other countries.2
1.1. Routes of Arsenic Exposure
1.1.1. Arsenic in Drinking Water
Arsenic is a naturally occurring metalloid found in insoluble forms in soil, which can leach into groundwater once mobilized.3 It is estimated that over 140 million people in at least 70 countries are exposed to > 10 μg/L of As via drinking water, the WHO4 and United States (US) Environmental Protection Agency (EPA)5 safety standard. Much of the global burden of As-related morbidity and mortality is carried by those countries that are the most severely affected by As exposure, including Bangladesh, Cambodia, China, India, Myanmar, Nepal, Pakistan, Taiwan, Argentina, and Vietnam.6 In Bangladesh, large-scale exposure to As through drinking water began in the 1970s. The United Nations Children’s Fund (UNICEF) and the Department of Public Health Engineering installed tube wells to reduce infant mortality related to diarrheal disease connected to contaminated surface water. Arsenic contamination of well water was not identified until the 1990s, with concentrations that ranged from below the limit of detection (LOD) to over 1,000 μg/L.7 Elevated As concentrations in groundwater are also found in the Western, Midwestern, and Northeastern US.8 In the US, it has been estimated that about 730,0009 people are exposed to As-contaminated water > 10 μg/L served by community water systems, and more than 2.1 million10 people exposed to water As > 10 μg/L from private wells. This is related to challenges in implementing the drinking water standard and the use of private wells, which are not included in EPA drinking water regulations.11
1.1.2. Arsenic in Food and Other Beverages
In regions where drinking water As concentrations are low, ingestion of As through As-contaminated food and beverages can represent a relatively large proportion of As exposure. In human experimental12,13 and epidemiological studies,14,15 consumption of rice and rice products has been associated with increased urinary As. Arsenic found in rice is primarily arsenate (InAsV), arsenite (InAsIII), or dimethylarsinic acid (DMAsV).16 Consumption of rice-based products is a particular concern for infants and children who have significantly higher relative As intake as a result of a higher per-body-mass food intake and a less varied diet.17 However, in prospective cohort studies, rice consumption has not been associated with increased risk for cancers or CVD, although these studies were based on food frequency questionnaires and did not measure As concentrations in rice or As biomarkers.18–20 Arsenic has also been detected in apple juice21 and some red wines,22 with As concentrations exceeding 10 μg/L. Arsenic concentrations in common foods and beverages assessed by the US Food and Drug Administration (FDA) and EPA have been previously summarized.1
1.1.3. In Utero and Early Life Arsenic Exposure
Arsenic species can readily pass through the placenta, and maternal blood concentrations of total As and As species are highly correlated with those of umbilical cord blood.23,24 Arsenic does not readily enter the mammary gland; consequently, low concentrations of As, e.g., 1 to 3 μg/L, have been found in breast milk.24–26
1.1.4. Arsenic Exposure via Inhalation
Arsenic can be absorbed by the respiratory tract. This is a concern for occupations that involve As use or production such as smelting, and individuals living in nearby communities.27 Inhalation exposure may also be due to burning of As-containing biomass, as occurs in regions where cattle are exposed to As through drinking water and consumption of contaminated rice straw.28
1.2. Health Effects of Arsenic Exposure
1.2.1. Health Effects of Arsenic Exposure in Adults
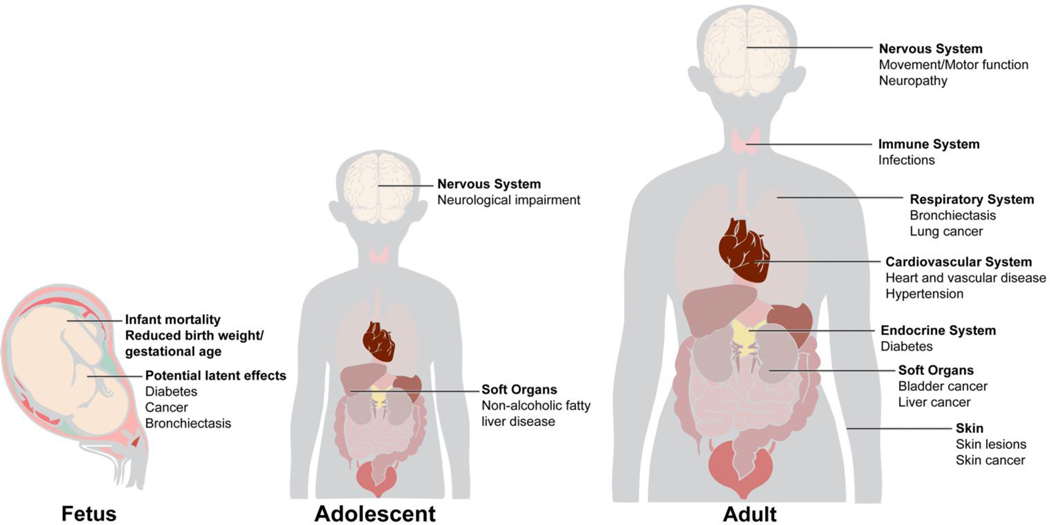
Arsenic is a Group 1 human carcinogen and toxicant, and affects numerous organs.29 Exposure to As is associated with an increased risk of health outcomes such as skin lesions (melanosis, leukomelanosis, and keratosis); cardiovascular disease (CVD); impaired intellectual function; diabetes and diabetes-related outcomes;30 and cancers of the bladder, lung, liver and skin (Figure 1).31–33 Increased risk for several As-related cancers, e.g. lung and bladder cancer, have been shown to persist long after exposure to As has been reduced.34 There is some evidence suggesting that As exposure is also associated with increased risks of breast35 and prostate36 cancers. In Taiwan, chronic exposure to As is associated with blackfoot disease.37
Figure 1.
Arsenic Target Tissues and Comorbidities. Chronic As exposure has been associated with increased risk of skin lesions (melanosis, leukomelanosis, and keratosis), cardiovascular disease, impaired intellectual function, inflammation, diabetes, and cancers. Ingested As accumulates in multiple tissues including the spleen, liver, lungs, bladder, esophagus, skin, and bone marrow.
1.2.2. Health Effects of Arsenic Exposure in Children and Adolescents
Arsenic exposure in childhood and adolescence has been linked to non-alcoholic fatty liver disease,38 atherogenic lipid profile39 and increased lifetime risk of cancer.40 A potential association between arsenic exposure and high blood pressure has also been observed in a cross-sectional study of adolescents.41 Arsenic exposure has been also associated with adverse neurological effects in adolescents42 and cognitive effects in children.43,44
1.2.3. Health Effects of In Utero Arsenic Exposure
Arsenic exposure, as measured by maternal urinary As, has been associated with decreased birth weight and gestational age, and increased infant mortality,45 although results from epidemiological studies have been inconsistent.46–48 A recent meta-analysis found a negative association between maternal As exposure and birth weight (summary regression coefficient = −25.0 g; 95% CI: −41.0, −9.0).49 Epidemiological studies have also found in utero As exposure to be associated with impaired immune function.50 In utero and early-life exposure has been associated with increased mortality from chronic renal disease and lung, bladder, liver, and laryngeal cancers in adulthood.51,52 Additionally, As exposure may play a role in the development of gestational diabetes mellitus (GDM).53
1.3. Arsenic Methylation and Elimination
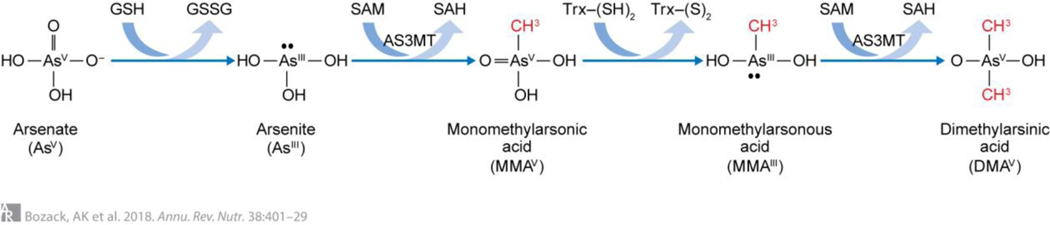
Arsenic in drinking water is primarily present as InAs, either as arsenate or arsenite. Once ingested, InAs is methylated in a process that facilitates urinary As elimination.54 As described by Challenger in 1945, As undergoes sequential reduction and oxidative methylation reactions (Figure 2).55 OCM is essential for the synthesis of the methyl donor, S-adenosylmethionine (SAM). Arsenic-3-methyltransferase (AS3MT) catalyzes the transfer of a methyl group derived from SAM to InAsIII, forming monomethylarsonic acid (MMAsV).55–57 MMAsV is then reduced to monomethylarsonous acid (MMAsIII), an intermediate with very high cytotoxicity and genotoxicity.58–60 AS3MT then catalyzes a second methylation reaction, forming DMAsV.55–57 DMAsV is substantially less toxic than MMAsIII or InAsIII + V, and is rapidly excreted in urine.60 The role of As methylation in urinary As elimination is particularly evident in mice deficient in AS3MT; these mice accumulate As within tissues at levels 16–20 times greater than wild-type mice resulting in severe systemic toxicity.61,62
Figure 2.
Arsenic Methylation. According to the Challenger pathway, arsenic (+3 oxidation state) methyltransferase (AS3MT) catalyzes the oxidative methylation of arsenite using s-adenosylmethionine (SAM) as the methyl donor, forming methylarsonic acid (MMAV), and s-adenosylhomocysteine (SAH). MMAV is then reduced to methylarsonous acid (MMAIII) before a subsequent oxidative methylation step yielding dimethylarsinic acid (DMAV) and SAH.
Total urinary As adjusted for urinary dilution is a well-established biomarker of As exposure in humans. Arsenic methylation patterns are analyzed as the relative distribution (%) of InAs, MMAs and DMAs in urine. Analytical advances have also allowed for measurement of total As and As species in blood. Although the distribution As species differs in urine and blood, urine and blood As species concentrations are highly correlated (Spearman ρ range: 0.68 – 0.81).63 However, the half-life of DMAs in blood is relatively short, resulting in considerably lower %DMAs and higher %MMAs in blood than urine.63
Trivalent arsenicals are readily oxidized to pentavalent forms by environmental oxygen, and therefore it is difficult to distinguish between the valence states particularly in studies where biological samples are stored for prolonged periods. Most methods cannot measure detectable levels of MMAsIII or DMAsIII,64 thus, this was not traditionally done in epidemiological studies. While InAsIII elutes as a separate peak from InAsV, most human studies report levels of InAs as InAsIII+V.
1.4. Influence of Arsenic Methylation on Arsenic Toxicity
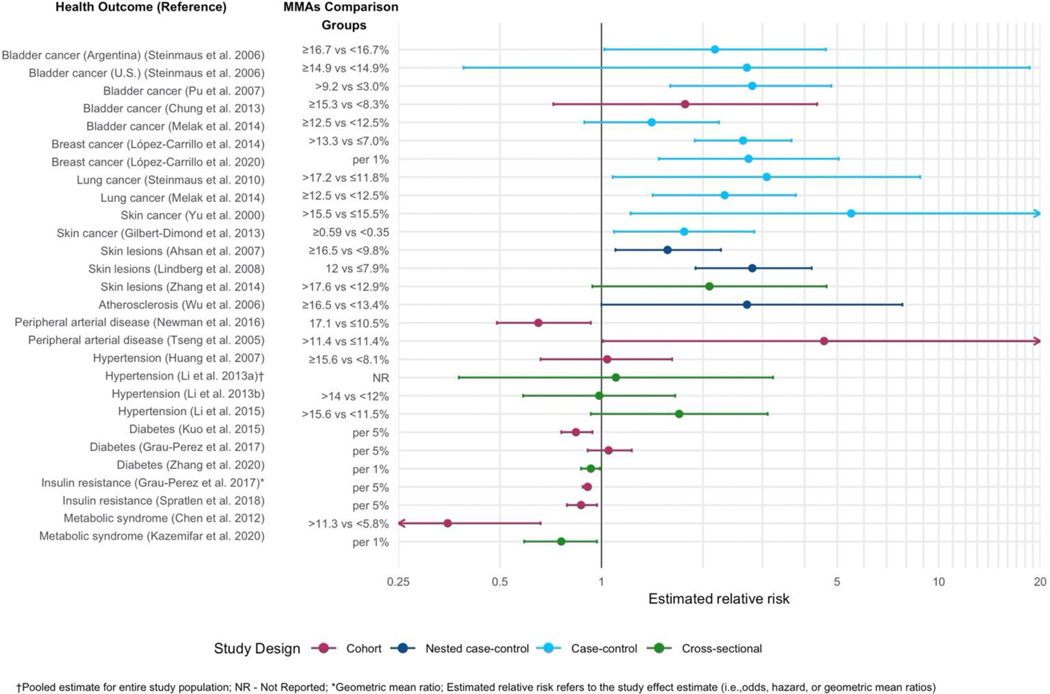
Arsenic species vary considerably in their toxicity, and trivalent arsenicals are considerably more toxic than pentavalent species. Studies in human cell lines have determined the most to least toxic forms to be: MMAsIII, DMAsIII, InAsIII, InAsV, MMAsV and DMAsV.58,65 Epidemiological studies and a meta-analysis of cancer risk66 have found positive associations between %MMAs in urine and risk of cancers of the bladder,67–70 breast,35,71 lung,69,72 and skin,73,74 in addition to skin lesions,75–77 atherosclerosis,78 and peripheral arterial disease,79,80 although for the latter there were some inconsistencies. In Figure 3, we plotted the estimated relative risk for disease from several of these studies illustrating that the effect estimates associated with higher %MMAs in urine for these health outcomes range from approximately 1.6 for skin lesions to as high as 5.5 for skin cancer. Note that this is not a comprehensive representation of all studies linking decreased As methylation to health outcomes. Not all studies reported %MMAs, e.g. some studies reported increased risk of bladder cancer in relation to increased primary methylation,81 decreased secondary methylation index (DMA/MMAs), and/or decreased %DMAs in relation to e.g. risk for skin lesions82 and urothelial carcinoma83. In contrast, associations with hypertension84–87 were generally null, and more recently, inverse associations have been found between %MMAs in urine and risk of diabetes, and diabetes-related outcomes including metabolic syndrome and insulin resistance, with relative risks ranging from 0.35 to 1.05.30,33,88–91 These findings are also consistent with an inverse association between %MMAs and adiposity (not shown in the figure).88,91–96 However, the relationship between As methylation and metabolic outcomes is not fully understood, and we cannot at present fully rule out reverse causality or confounding. A limitation common to most of the studies in Figure 3 is that they employ prevalent cases35,67–69,71–74 and therefore temporality cannot be firmly established; however, experimental data58,61,62,65 and nested case-control studies in which As species were measured prior to disease onset75,76 also support the toxicity of higher %MMA and risk for multiple As-related health outcomes.
Figure 3.
Summary plot of estimated relative risks and 95% confidence intervals for adverse health outcomes reported to be associated with MMAs in urine (Citations provided in Section 1.2).
Efficiency of As methylation is variable within and across species, individuals, and populations. In humans, genetic variation is a strong determinant of As methylation capacity95,97–100 and As-related health outcomes (e.g., skin lesions and cancer).75,100
1.5. Mechanisms of Action of Arsenic Influenced by One-Carbon Metabolism
Arsenic’s mechanisms of action are likely multifactorial and are not completely understood. Biological mechanisms involved in As-related health outcomes may involve DNA repair, oxidative stress, cell cycle disruption, endocrine disruption, inhibition of thymidylate synthesis, epigenetic dysregulation, microbiome disruption, cytotoxicity, and proliferative regeneration.101–110 We briefly summarize mechanisms which are likely influenced by OCM, recognizing that the role of nutrition on these processes is often overlooked.
1.5.1. Gut Microbiome Disruption
Disruption of the gut microbiome (GM) has been shown to influence OCM in mouse models. In GM-disrupted mice, there was downregulation of the hepatic expression of OCM-related genes, including folate receptor 2 (FOLR2), betaine homocysteine methyltransferase (BHMT), and methylene tetrahydrofolate reductase (MTHFR). Additionally, GM-disrupted mice exposed to As had a significant decrease in hepatic SAM levels compared to GM-disrupted mice that were not exposed to As. GM disruption may potentially influence the biotransformation of As through impacts on OCM.108 GM disruption was also found to increase the toxic effects of As as indicated by increased mortality and alteration in expression of cancer-related genes.108,109
There is also evidence that the biotransformation of As in mammals can be influenced by gut bacteria. GM composition, influenced by a wide range of genetics or pathogenic infections, has been associated with As methylation patterns, indicative of a possible role of GM phenotypes in As methylation.111,112
1.5.2. Epigenetic Dysregulation.
Early-life exposure to As increases the risk of diseases later in life, with As-related health effects persisting long after exposure has ceased.113,114 Studies in animals and human cells strongly suggest that the etiology of As-related diseases, such as lung cancer, involves epigenetic dysregulation (e.g.115,116), which could be influenced by nutritional status. Studies of epigenetic dysregulation associated with chronic As exposure have been previously reviewed.105,117,118 In summary, As exposure has been shown to induce genome-wide DNA hypomethylation in cell-culture studies, likely contributing to genomic instability.119 In epidemiological studies, As has been associated with alterations in global DNA methylation,120–123 loci-specific DNA methylation,117,124–127 global 5-hydroxymethylcytosine (a DNA demethylation intermediate product),128 and with histone modifications.129 In several studies of Bangladeshi adults, we found that As was positively associated with peripheral blood mononuclear cell (PBMC) global DNA methylation, a potential compensatory mechanism to prevent hypomethylation, and an effect that was modified by folate status.120,121,130 Epigenome-wide association studies of As exposure have found associations with DNA methylation levels at individual loci, although a common signature of As exposure has not been identified.117,120,121,130–132 Although few epidemiological studies have investigated mediation of the association As and health outcomes by epigenetic dysregulation, a study conducted in Bangladesh found an association between As exposure and methylation of four CpG sites and risk for skin lesions (Bonferroni-adjusted significance threshold: PBonferroni <1×10−7).126
2. Nutritional Influences on Arsenic Methylation via One-Carbon Metabolism
2.1. Nutrition and One-Carbon Metabolism
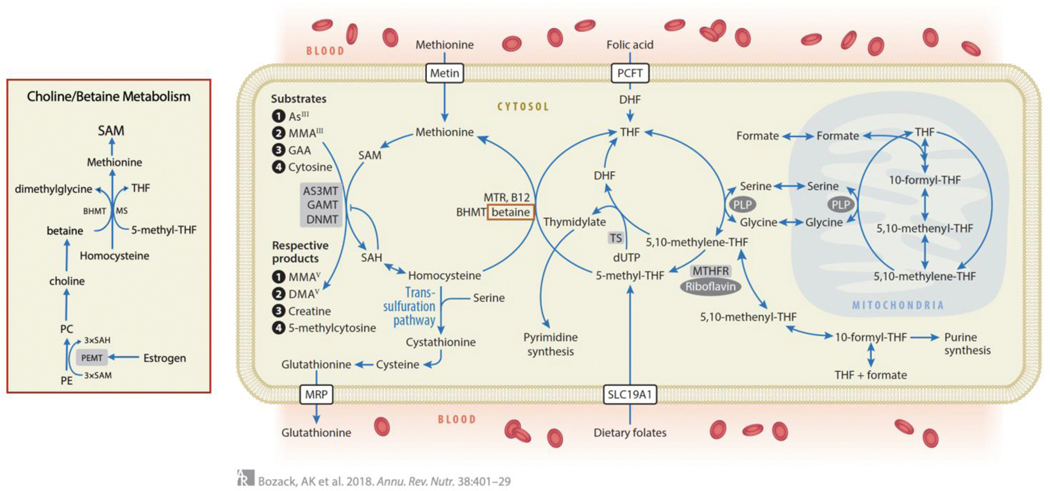
The synthesis of SAM through OCM is essential for the methylation of hundreds of substrates including As, DNA, RNA, histones, lipids, small molecules and proteins133 (Figure 4). Critical micronutrients involved in OCM include folate, vitamin B12 (cobalamin), betaine, choline, vitamin B6 (pyridoxal phosphate), riboflavin, and niacin. In the folate cycle, a methyl group is transferred from serine to tetrahydrofolate (THF) by serine hydroxymethyltransferase (SHMT) to form 5,10-methylene-THF, which is subsequently reduced to 5-methyl-THF by MTHFR. 5-methyl-THF is the most prevalent form of naturally occurring dietary folate.134 Fortification of foods uses the synthetic and chemically stable form of folate, folic acid (FA), which can be reduced by dihydrofolate reductase to THF. The methyl group of 5-methyl-THF is transferred to homocysteine (Hcy) by methionine synthase utilizing B12 as a cofactor, generating methionine and THF. For this reaction, an alternative methyl donor is betaine, which is obtained exogenously from the diet or endogenously synthesized from choline. Methionine is then activated by methionine adenosyltransferase to form SAM.135 Along with the methylated products formed from all methylation reactions, S-adenosylhomocysteine (SAH) is also produced, and is hydrolyzed to form Hcy. SAH is a potent product inhibitor of most methyltransferase enzymes; therefore, 5-methyl-THF is critical for pulling the pathway forward. SAM concentrations are tightly regulated by numerous long-range allosteric interactions. Under conditions of folate deficiency where the pool of SAM may become limiting, methylation reactions, such as As methylation, may decrease. However, other critically essential reactions, such as DNA methylation, have very low KMs for SAM and can thus operate at full capacity even when SAM concentrations are quite low. Although OCM requires many micronutrients, folate is a primary nutritional factor involved in influencing circulating Hcy concentrations. For example, in a cross-sectional survey of Bangladeshi adults with high prevalence of elevated Hcy, 15% and 5% of the variance in Hcy were explained by plasma folate and B12, respectively.136 However, the relative contribution of micronutrients to the variance in Hcy likely varies by additional factors such as age, diet, sex and geographical region.
Figure 4.
One-Carbon Metabolism (OCM). In the folate cycle, a methyl group is transferred from serine to THF by serine hydroxymethyl-transferase, with pyridoxal phosphate (PLP) as a coenzyme, forming 5,10-methylene-THF. This is either used for the synthesis of thymidylate or is reduced to 5-methyl-THF. 5-methyl-THF is the most prevalent form of naturally occurring dietary folate. Fortification of foods uses the synthetic and chemically stable form of folate, folic acid (FA), which can be reduced by dihydrofolate reductase to THF. The methyl group of 5-methyl-THF is transferred to homocysteine in a reaction catalyzed by methionine synthase (MTR) and utilizing B12 as a cofactor, generating methionine and THF. Alternatively, in the liver, betaine can donate a methyl group for the remethylation of homocysteine in a reaction catalyzed by betaine homocysteine methyltransferase (BHMT). Methionine adenosyltransferase enzymes activate methionine to form S-adenosylmethionine (SAM), the methyl donor for numerous acceptors, including arsenicals, guanidinoacetate (GAA, the precursor to creatine), and DNA, in reactions that involve substrate-specific methyltransferase enzymes. These methylation reactions generate the methylated products and S-adenosylhomocysteine (SAH), a potent product inhibitor of most methyltransferases. SAH is hydrolyzed to generate homocysteine which is either remethylated to regenerate methionine or is directed to the transsulfuration pathway and to glutathionine synthesis. (Inset) Betaine can either be obtained from the diet or can be generated through endogenous synthesis from choline. However, because choline is synthesized de novo through a pathway in which PEMT catalyzes three sequential SAM-dependent methylation reactions, endogenous choline synthesis is a significant consumer of SAM. PEMT uses three molecules of SAM to synthesize phosphatidyl choline (PC) from phosphatidylethanolamine (PE), and is transcriptionally regulated by estrogen.
2.1.1. Major Consumers of SAM.
The two major consumers of SAM are guanidinoacetate methyltransferase (GAMT), important to creatine synthesis, and phosphatidylethanolamine methyltransferase (PEMT), important to phosphatidylcholine (PC) synthesis. In omnivores, about half of creatine requirements are obtained through dietary intake, primarily through the consumption of meat.137 Therefore, urinary creatinine concentrations reflect both creatine intake through the diet and endogenous synthesis of creatine.137 Approximately 50% of all SAM-derived methyl groups are consumed by the methylation of guanidinoacetate (GAA), the final step of creatine synthesis.138,139 Dietary intake of creatine inhibits the synthesis of GAA, and consequently inhibits creatine biosynthesis; this has been shown to spare methyl groups and lower Hcy in rodents.140–142 The other major consumer of SAM, PEMT, uses three molecules of SAM to synthesize PC from phosphatidylethanolamine, and is transcriptionally regulated by estrogen.143 Other methyltransferase enzymes, including AS3MT, consume only a small fraction of SAM. For example, based on one-carbon kinetics,144 we estimated that even at high levels of As exposure (500–1,000 μg/L), methylation of 80% of a daily dose of InAs to DMAs would require approximately 50 μmol SAM, or roughly 2–4% of the SAM normally turning over in a well-nourished adult per day.145
2.2. One-Carbon Metabolism and Arsenic Methylation in Animal Models
Animal models provided the first evidence that As methylation and toxicity were influenced by nutritional regulation of OCM. Vahter & Marafante found that rabbits fed diets deficient in methyl donors (i.e., methionine, choline, or protein) had significantly lower total urinary As and DMAs excretion, and increased uptake of As in tissues.146 Similar findings were also reported in mice.147 The effect of folate on As methylation and neural tube defects (NTDs) was subsequently tested by Finnell’s group using mice nullizygous for several folate-binding proteins involved in cellular uptake of folate from the circulation, e.g., Folbp-1 and −2, and/or reduced folate carrier proteins.148–151 Folbp-1 knockout mice were more susceptible to As-induced NTDs.148 Mice exposed to As and maintained on folate deficient diets had a reduction in urinary DMAs excretion in all genotypes assessed.148–151 While the link between As exposure and NTD risk has not yet been firmly established in humans, a study by Mazumdar et al. in Bangladesh found that the efficacy of FA in preventing NTDs was reduced by As exposure.152 Among mothers with low folate status, As exposure was associated with a greater risk of having an infant with myelomeningocele.153
Animal models have limited utility for studying the effects of OCM-related micronutrients on As methylation efficiency for four main reasons: (1) As methylation capacity varies drastically between species; (2) humans are more likely to develop As-related cancers than animals; (3) rodents are unlikely to develop folate deficiency, and (4) it is difficult to replicate chronic (i.e., decades-long), low-dose As exposure in rodent studies. There is new promise for a resolution to the problem of high As methylation efficiency among rodents with the recent generation of a mouse model carrying a humanized BORCS7/AS3MT locus by syntenic replacement, as the As methylation profile of these mice more closely resembles that of humans.154
2.3. One-Carbon Metabolism and Arsenic Methylation in Humans
This section will summarize the literature on the influence of OCM-related nutrients on As methylation efficiency throughout the life course, with a primary focus on folate. The research conducted in humans on the associations between creatine, vitamin B12, choline, and betaine with As methylation profiles is also summarized below. All results are significant with p-values < 0.05 unless otherwise stated. Although the influence of these OCM-related micronutrients on As methylation are not as well studied as folate in regard to As methylation, they provide potential avenues for future research.
2.3.1. Folate
Isolated case reports provided the earliest human data on the role of folate in As-related toxicity. For example, a 1965 report in Lancet described an 18-year-old female treated for psoriasis with Fowler’s solution who developed clinical signs of As-related toxicity. She was found to be folate deficient and her symptoms responded to intramuscular FA treatment.155 Another case study presented a 16-year-old girl with severe MTHFR deficiency and homocystinuria exposed to an As-containing pesticide that developed severe symptoms of As-related toxicity.156 Epidemiologic studies of As exposure and folate status (described below) emerged later.
2.3.1.1. Folate and Arsenic Methylation during Adulthood
The relationship between folate and As methylation capacity among adults has been studied in observational and intervention studies, as has previously been reviewed,1 and is here updated in Table 1. In 2002, a cross-sectional study by Smith’s group investigated As-contaminated drinking water of 11 families in Chile (N=44). Arsenic methylation was weakly correlated between fathers and mothers; however, adjustment for plasma folate and Hcy increased the strength of these the correlations, suggesting these factors account for some of the variation in As methylation capacity.157 In an early case-control study in West Bengal, India, Smith’s group found that higher serum folate was associated with significantly lower %MMAs.158 In 2005, another study conducted by Smith’s group in the western US assessed diet using questionnaires, and found that lower dietary protein, iron, zinc, and niacin were associated with higher urinary %MMAs and lower %DMAs.159 Unlike the previous studies conducted by Smith’s group, no significant associations with dietary folate intake were found; however, these study participants likely had fairly high folate intake due to the 1998 US mandate for the FA fortification of foods.
Table 1.
Summary of epidemiological studies on folate and arsenic methylation
| Study | Design | Population | Measure of folate status | Summary of results related to folate* | Summary of results related to other OCM micronutrients* |
|---|---|---|---|---|---|
| Mitra et al. 2004;196 Basu et al. 2011158 | Case-control study for As-induced skin lesions | Individuals in West Bengal, India with drinking water As <500 μg/L (N=405) | 24-hr dietary recall; serum folate | • Lowest quintile of folate intake was not significantly associated with skin lesions risk • Highest to lowest quintile of folate intake had a significant positive trend in OR for skin lesions • Compared to the highest tertile, the lowest tertile of serum folate was associated with lower %InAs and higher %MMAs in urine |
• Lowest quintile of animal protein intake was associated with risk of skin lesions • Highest to lowest quintile of animal protein intake had a significant positive trend in OR for skin lesions • Compared to the highest tertile, the lowest tertile of urine creatinine was associated with higher %InAs, and lower %MMAs and %DMAs in urine • Lowest tertile of plasma Hcy was associated with lower urine %MMAs compared to the highest tertile • Compared to the highest tertile, the lowest tertile of riboflavin intake was associated with lower %InAs and higher %DMAs in urine • Compared to the highest tertile, the lowest tertile of animal protein intake was associated with lower urine %MMAs |
| Steinmaus et al. 2005159 | Crosssectional study | Individuals in Western U.S. (N=87) | Dietary questionnaire | • Folate intake was not significantly associated with urine As species proportions | • Compared to highest quartile, the lowest quartile of protein intake was associated with higher %MMAs and lower %DMAs in urine |
| Gamble et al. 2005160 | Crosssectional study | Individuals in Bangladesh (N=300) | Plasma folate | • Plasma folate was associated with lower %InAs and %MMAs, and higher %DMAs in urine | • Total homocysteine was associated with higher %MMAs and lower %DMAs in urine • Cysteine was associated with lower %InAs and higher %MMAs in urine • Urinary creatinine was associated with lower %InAs and higher %DMAs in urine |
| Gamble et al. 2006;161 Gamble et al. 200763 | Randomized controlled trial | Folate deficient individuals in Bangladesh (N=300) | Supplementatio n of 400 μg FA/day for 12 weeks | • Compared to placebo, FA treatment was associated with a greater decrease in %InAs and %MMAs, and increase in %DMAs in urine • Compared to placebo, FA treatment was associated with a greater decrease in total blood As and blood MMAs concentrations |
• Urinary creatinine was associated with lower %InAs and higher %DMAs in urine |
| Li et al. 2008176 | Cohort study | Pregnant women in Bangladesh (N=864) | Plasma folate at gestational week 14 | • Compared to the highest tertile, the lowest tertile of plasma folate was associated with higher urine %InAs among women with urine As >209 μg/L • Compared to the highest tertile of plasma folate, B12, and zinc, the lowest tertile of plasma folate, B12, and zinc were associated with higher urine %InAs and primary methylation index |
• Urinary creatinine was associated with lower %iAs, and higher %DMAs, primary methylation index, and secondary methylation index |
| Pilsner et al. 2009130 | Nested case-control study for As-induced skin lesions | Individuals in Bangladesh (N=548) | Plasma folate | • Compared to high folate, low folate (< 9 nmol/L) was associated with increased risk of skin lesions • Compared to the referent group of low Hcys + high folate, low homocysteine + low folate, hyperhomocysteinemia + high folate, and hyperhomocysteinemia + low folate were all associated with increased risk of skin lesions |
• Compared to low homocysteine, hyperhomocysteinemia was associated with increased risk of skin lesions • A fold increase in urinary creatinine was associated with decreased risk of skin lesions • Compared to the lowest quartile of urinary creatinine, the second and third quartile of urinary creatinine were associated with decreased risk of skin lesions |
| Gardner et al. 2011178 | Cohort study | Pregnant women in Bangladesh (N=324) | Plasma folate | • In multivariate mixed effects linear models, plasma folate was not associated with change in urine As proportions during pregnancy | • In multivariate mixed effects linear models, plasma B12 was not associated with change in urine As proportions during pregnancy |
| Peters et al. 2015;162 Bozack et al. 2017163 | Randomized controlled trial | Individuals in Bangladesh (N=610) | Supplementatio n of 400 μg FA/day, 800 μg FA/day, or 400 μg FA + 3g creatine/day for 12 weeks | • Compared to placebo, 800 μg FA/day treatment was associated with a greater decrease in total blood As • Compared to placebo, FA treatment was associated with a greater decrease in %InAs and %MMAs, and increase in %DMAs in urine |
• Compared to placebo, creatine treatment was associated with a greater decrease in urine %MMAs |
| López-Carrillo et al. 2016165 | Crosssectional study | Women in Northern Mexico (N=1,027) |
Dietary questionnaire | • Compared to low folate intake, High folate intake ≥ 400 μg/day was associated with lower urine %InAs and higher total methylation | • B12 intake was associated with lower urine %InAs and higher urine %DMAs, second methylation, and total methylation • Choline intake was associated with lower urine %InAs and higher urine %DMAs, second methylation, and total methylation • Methionine intake was associated with lower urine %InAs and higher urine %DMAs, second methylation, and total methylation |
| Kurzius-Spencer et al. 2017166 | Crosssectional study | Adults and children in the U.S. (N=2420) | Red blood cell and serum folate; 24-hour dietary recall | • Folate intake was associated with lower urine %InAs and higher urine %DMAs among adults • Red blood cell and serum folate were not associated with urine As species proportions • In multivariate models, red blood cell folate was associated with lower urine %InAs • In multivariate models, folate intake and serum folate were not associated with urine As species proportions or secondary methylation index |
• Dietary B6 was associated with lower urine %InAs among adults • Plasma Hcy was associated with higher urine %MMAs and lower secondary methylation index among children • In multivariate models, urinary creatinine was associated with lower urine %InAs and %MMAs, and higher urine %DMAs and secondary methylation index • In multivariate models, B12 intake was associated with higher urine %InAs and lower urine %DMAs • In multivariate models, B6 intake was associated with lower urine %InAs • In multivariate models, plasma Hcy was associated with higher urine %MMAs, and lower urine %DMAs and secondary methylation index |
| Spratlen et al. 2017167 | Cohort study | American Indians (N=405) | Dietary questionnaire | • In fully adjusted models, folate intake was not associated with urine As species proportions • Compared to low folate intake and low B6 intake, high folate intake and high B6 intake were associated with lower %InAs and higher %DMAs in urine |
• Compared to the lowest tertile, the highest tertile of B2 intake was associated with lower %MMAs and higher %DMAs in urine • Compared to the lowest tertile, the highest tertile of B6 intake was associated with lower %InAs and %MMAs, and higher %DMAs in urine • First principal component of OCM nutrients (representing intake of all OCM nutrients) was associated with lower %InAs and %MMAs, and higher %DMAs in urine |
| Laine et al. 2018177 | Cohort study | Pregnant women and infant pairs in US (N=188) | Cord serum folate |
• Compared to infants born to folate replete mothers, infants born to mothers in the lowest tertile of serum folate had significantly higher mean levels of %MMA in cord serum | • Higher maternal Hcys was positively associated with maternal total urinary and cord serum As, and with %MMAs in cord serum |
| Bozack et al. 2019;164 Bozack et al. 2019181 | Randomized Control Trial | Adults in Bangladesh (N=622) | Plasma folate | • Relative to the placebo group, the 400- and 800-μg FA treatment groups had increases in As methylation capacity between baseline and week 12, as measured by decreases in urinary %InAs and %MMAs, and increases in urinary %DMAs | • No differences were found in treatment effects between the 400 μg FA and creatine + FA groups. |
| Saxena et al. In Press.173 | Crosssectional study | Adolescents in Bangladesh (N=679) | RBC and plasma folate |
• Plasma folate was negatively associated with %InAs and positively associated with %DMAs in male participants |
• In male participants, Hcys was positively associated with %InAs • In female participants, plasma Hcys was inversely associated with %MMA |
Except where otherwise indicated, all results were significant at P < 0.05.
To better understand the role of OCM nutrients such as folate in As methylation and toxicity, our group has conducted multiple studies in a Bangladeshi population with chronic As exposure through drinking water. In 2006, we reported the results of a cross-sectional study in Bangladesh, in which we observed that plasma folate was negatively associated with %MMAs and positively associated with %DMAs in urine.160 We went on to conduct a randomized, double-blind, placebo-controlled trial of folate-deficient adults (plasma folate <9 nmol/L) to study the effect of 400-μg FA per day (the US recommended dietary allowance) on As methylation. The treatment group had significantly larger decreases in %InAs and %MMAs and larger increases in %DMAs in urine following 12 weeks of supplementation compared to placebo, with treatment effects found as early as one week of intervention.161 Furthermore, compared to the placebo group, total blood As and blood MMAs concentrations were lowered following FA supplementation by 14% and 22%, respectively.63 In the Folic Acid and Creatine Trial (FACT), a randomized controlled trial (RCT) in Bangladesh, participants were selected independent of folate status, and received 400 μg FA, 800 μg FA, 3 g creatine, 3 g creatine + 400 μg FA, or placebo daily for 24 weeks, including 12-week wash-out period where half of the FA-treated groups were switched to placebo to evaluate the effects of supplementation cessation on As methylation. Each participant received an As-removal water filter. After 12 weeks, the treatment group receiving 800 μg FA per day had a larger decrease in total blood As than the placebo group.162 Additionally, between baseline and week 12, all FA treated groups had increases in As methylation capacity, i.e., decreases in urinary %InAs and %MMAs, and increases in urinary %DMAs, relative to the placebo group.163,164 Further emphasizing the importance of continuous adequate folate nutritional status, FACT found that following the 12-week wash-out period, proportions of As species in urine reverted to pre-intervention levels. The results of these studies have important policy implications, as food FA-fortification programs may be necessary to achieve a sustained effect on folate status and improved As methylation capacity in As-exposed regions.
Our findings of the influence of folate on As methylation capacity have been confirmed in epidemiological studies including those with populations exposed to lower levels of As. A cross-sectional study in Mexico used a food frequency questionnaire to estimate the micronutrient intake and found that higher folate intake was significantly associated with lower %InAs and a higher ratio of DMAs/InAs.165 An analysis of the 2003–2004 US National Health and Nutrition Examination Survey (NHANES) found that in unadjusted models, dietary folate intake was negatively associated with %InAs and positively associated with %DMAs in urine, and in adjusted models, red blood cell (RBC) folate was negatively associated with urinary %InAs.166 In the Strong Heart Study (SHS), a population-based prospective cohort study of American Indian adults residing within 13 tribal communities with low-to-moderate As exposure, a food frequency questionnaire was used to estimate nutrient intake in American Indians, and high combined intake of folate and vitamin B6 was associated with lower urinary %InAs and higher %DMAs.167 In contrast, no significant associations were found between dietary folate intake and As methylation efficiency in a study in Bangladesh. However, accurate measurement of dietary folate intake is difficult in Bangladesh due to local traditions of prolonged cooking that degrades naturally occurring food folate.168 This underscores the benefits of assessing circulating folate concentrations over that of dietary intake in order to more accurately assess folate nutritional status.
2.3.1.2. Folate and Arsenic Methylation during Childhood and Adolescence
The literature regarding OCM and As methylation during childhood and adolescence is limited. Children are reportedly more efficient at methylating As than adults.169–172 In a cross-sectional study of 6-year-old children in Bangladesh (N=165), plasma folate was inversely correlated with %InAs and positively correlated with %DMA in urine. Although the latter did not achieve statistical significance, the magnitude of the association (r = 0.12, p = 0.14) was similar to that observed in cross-sectional studies of adults (r = 0.14, p < 0.05), and the study sample size of children was relatively small. In a study of 9-year-old children by Vahter’s group, plasma folate was inversely associated with %InAs in urine and positively associated with %DMA. While less data is available for adolescents, we recently conducted a cross-sectional study of Bangladeshis between the ages of 14–16. In males, plasma folate was negatively associated with %InAs and positively associated with %DMAs in urine, consistent with our previous studies in adults. While no significant associations between folate and urinary As species were observed among females, RBC folate was inversely associated with total blood As.173 While speculative, it is possible that these findings may be related to short term fluctuations in estrogen, as estrogen is known to influence both OCM and choline synthesis,174,175 and choline likely influences As methylation (see sections 2.1.1. and 2.3.4.). However, additional research in adolescents is needed to better understand these findings.
2.3.1.3. Folate and Arsenic Methylation in Prenatal Development and Early Life
Maternal OCM is altered during pregnancy due to increased requirements needed to support fetal development. Maternal plasma folate levels change considerably throughout pregnancy as folate is preferentially transferred to the fetus.175 Several studies have investigated the role of OCM-related micronutrients in the efficiency of As methylation in pregnant women. A cross-sectional study conducted by Vahter’s group that evaluated women at 14 weeks gestation in Bangladesh (N=753) found an inverse association between plasma folate and %InAs in urine. Compared to women that were sufficient in folate, vitamin B12, and zinc, those that were deficient in all three nutrients had significantly higher urinary %InAs and lower primary methylation indices.176 A study conducted by Laine et al. in a pregnancy cohort in Mexico, found that the distribution of As species in cord serum may be influenced by maternal OCM status: compared to infants born to folate sufficient mothers, those born to folate deficient mothers had significantly higher mean levels of %MMA in cord serum. Furthermore, higher maternal Hcys was positively associated with maternal total urinary and cord serum As, along with %MMAs in cord serum.177 A subsequent study conducted by Vahter’s group assessed Bangladeshi women at gestational weeks 8, 14, and 30. Although neither plasma folate nor B12 were associated with As species proportions, gestational week was significantly negatively associated with %InAs and %MMAs in urine, and positively associated with %DMAs.178 Due to dramatic changes in OCM during the course of pregnancy, it is difficult to interpret these observed associations between gestational week and As methylation. Further complicating the evaluation of OCM nutrient status during pregnancy, all women in this study received a daily supplement of 400-μg FA starting from week 14, potentially influencing As methylation efficiency.
2.3.2. Creatine and Creatinine (see also Section 2.1.1.)
Urinary creatinine is a degradation product of creatine that reflects both dietary creatine consumption and creatine synthesized endogenously. It is secreted into urine at a relatively constant rate over the course of a day and urinary creatinine concentrations are commonly used to adjust for urine dilution. Urinary creatinine has consistently been found to be negatively associated with urinary %InAs and positively associated with urinary %DMAs, as observed by members of our group160,161,170 and others.158,179 For reasons that remain somewhat unclear, urinary creatinine is a remarkably robust predictor of As methylation efficiency.
The role of OCM on creatine synthesis at the “expense” of large amounts of SAM-derived methyl groups is described in Section 2.1.1. As dietary creatine, through meat or through dietary supplements, downregulates creatine synthesis, we hypothesized that creatine supplementation may facilitate As methylation by sparing methyl groups in our FACT study. We found that participants receiving 3 g of creatine per day (equivalent to about 1.5 times the normal daily creatine turnover for a 70-kg male) for 12 weeks lowered GAA as predicted, indicating that creatine supplementation downregulated creatine synthesis.180 Additionally, the creatine treatment group had a mean decrease of %MMAs in urine that exceeded that of the placebo group at weeks 6 and 12. However, changes in %InAs or %DMAs in the creatine treatment group were not significantly different than placebo.181
There are known metabolic interactions between folate and choline/betaine: e.g., when folate is low, choline/betaine are preferentially used to remethylate Hcy. In FACT analyses stratified by baseline choline status, creatine supplementation resulted in a decrease in the primary methylation index in the low choline strata but not in the high choline strata.182 This may be related to the fact that when choline status is low, PC synthesis by PEMT is upregulated, increasing consumption of SAM (see sections 2.1.1. and 2.3.4).183,184 It is also possible that creatine treatment effects were tempered by long-range allosteric regulation of OCM. Further research and consideration of alternative mechanisms may be required to fully understand the strong cross-sectional associations between As methylation and urinary creatinine. For example, urinary creatinine reflects meat and methionine intake, which could influence As methylation. The role of creatine/creatinine on As methylation may be multifactorial and warrants further study.
2.3.3. Vitamin B12 (cobalamin)
Methylcobalamin plays an essential role as a co-factor for methionine synthase, a critical step in the process of SAM synthesis, thus, one would predict that vitamin B12 status, like folate, would be positively associated with As methylation capacity. However, vitamin B12 has been inconsistently linked to As methylation. A rodent study found that exposing dams to As via drinking water induced fasting hyperglycemia and insulin resistance among male offspring; this phenotype was rescued with a folate/B12 supplemented diet during gestation. These findings suggest that prenatal exposure to As may have a sex-specific adverse effect on glucose metabolism. The supplemented diet only had marginal effects on As methylation in the dams when compared to dams receiving a folate/B12 adequate diet, but standard chow diets are high in folate and no folate-deficient group of dams was available for comparison.185
Associations between B12 and As methylation have also been investigated in epidemiological studies. In a cross-sectional study in Bangladesh that oversampled B12-deficient individuals (N=778), we found that plasma B12 concentrations were negatively associated with urinary %InAs, positively associated with %MMAs, and not associated with %DMAs.186 Surprisingly, an analysis of the 2003–2004 NHANES found that dietary B12 intake was positively associated with urinary %InAs and negatively associated with %DMAs, but found no association between biomarkers of B12 nutritional status, including plasma B12 and methylmalonic acid, and As methylation.166 Other studies have found dietary B12 to be either unrelated to As methylation profiles167 or to be associated with increased As methylation.165
We further assessed associations between B12 and As species in urine and blood in the FACT and the Folate and Oxidative Stress (FOX) studies. In baseline samples from FACT, we found that B12 had nonsignificant correlations with As species in urine and negative associations with the concentrations of As species in blood. Although B12 was positively associated with urinary %MMAs (Spearman correlation coefficient ρ=0.12, p=0.02) in the FOX study, the correlation with blood MMAs concentrations was null (M.V. Gamble, unpublished data). In addition, our group’s recent study in adolescents found B12 to have no significant associations with urinary As species.173
Inconsistencies in studies of B12 and As methylation may be related in part to the relatively low nutritional requirements for B12 (e.g., the US RDA for B12 is 2.4 μg/d and the US RDA for folate is 400 μg/d), and it takes considerably longer to become B12 deficient. In addition, dietary sources of B12, almost entirely meat, are very different from folate which is derived largely from vegetables and fortified grains. Consequently, deficiencies of these two micronutrients do not necessarily overlap and there are lifestyle factors that distinguish them from one another that could influence As methylation capacity. Currently, there are no published studies that have investigated the treatment effects of B12 supplementation on As methylation efficiency in exposed populations. Additional research is required to fully understand the role of B12 in As methylation.
2.3.4. Choline and betaine.
In the liver, the remethylation of Hcy to methionine can utilize a methyl group from 5-methyl-THF or from betaine (Figure 4) via betaine-homocysteine S-methyltransferase (BHMT). As ~30% of choline is synthesized de novo through a pathway in which PEMT catalyzes three sequential SAM-dependent methylation reactions, endogenous choline synthesis is a significant consumer of SAM.187 PEMT has eight estrogen response elements and is strongly upregulated by estrogen, contributing to important sex differences in OCM.175
There are relatively few epidemiological studies of choline and As methylation. A cross-sectional analysis (N=1,016) nested within the Health Effects of Arsenic Longitudinal Study (HEALS) cohort in Bangladesh, found a positive association between dietary choline intake and the secondary methylation index.168 In a study of Mexican women, López-Carrillo et al. found an inverse association between dietary choline and urinary %MMAs and positive associations with %DMAs and the secondary methylation index.165 Findings from these observational studies suggest that dietary betaine has a weaker association than dietary choline with As methylation. For example, in both the HEALS cohort and López-Carrillo et al. study, dietary betaine intake was not associated As methylation efficiency.165,168
We conducted an 8-week pilot intervention of choline (700 mg/day), betaine (1,000 mg/day) or choline + betaine supplementation in Bangladesh (N=60). Compared to placebo, groups receiving supplementation had significantly different within-person changes in urinary %MMAs and %DMAs (M.N. Hall & M.V. Gamble, unpublished data). While the sample size was small, results suggest that choline + betaine supplementation led to the largest decrease in urinary %MMAs and increase in %DMAs, supporting the hypothesis that As methylation is influenced by both dietary choline and betaine. However, choline supplementation also increased plasma tri-methylamine oxide (TMAO), which has been linked to CVD risk. While it is not clear if the association between TMAO and CVD is causal,188 this finding tempered our interest in larger intervention studies with choline supplementation.189
2.3.5. Other One-Carbon Metabolism Micronutrients
Riboflavin (vitamin B2) is a cofactor for methionine synthesis, while niacin (vitamin B3) is a precursor for nicotinamide adenine dinucleotide (NAD), which is involved in OCM, other biosynthetic pathways, energy metabolism, and safeguards against ROS.190 In a random sample from the HEALS cohort (N=1041), increase in dietary riboflavin intake was inversely associated with urine %InAs, positively associated with %MMAs, and was not associated with %DMAs. Additionally, increase in dietary intake of niacin was associated with higher ratios of DMAs/MMAs.168 While these results suggest that niacin and/or riboflavin intake may influence As methylation capacity, no intervention studies have tested these hypotheses.
2.3.6. Impact of One-Carbon Metabolism-Related Polymorphisms
Several studies have been conducted to investigate the effect of OCM-related variants on As methylation. A case-control study of skin lesions in Bangladeshi adults (N=594 cases and 1,041 controls) led by H. Ahsan investigated the effect of two SNPs in MTHFR (rs1801133 and rs1801131); neither risk for skin lesions nor As methylation profiles differed significantly between haplotypes or diplotypes.75 In the Gene Environment Nutrient Interactions (GENI) study, we genotyped 26 SNPs in 13 OCM-related genes in a nested case-control study of incident skin lesions in Bangladesh (876 matched pairs).82 Although SNPs in MTHFR, methionine synthase (MTR), and serine hydroxymethyltransferase 1 (SHMT1) were nominally significant with urine As species proportions, no SNPs were statistically significant after FDR correction. In models predicting skin lesion risk, nominally significant interactions were found for three SNPs [rs1540087 and rs7109250 in folate receptor 1 (FOLR1) and rs1801394 in methionine synthase reductase (MTRR)] with water As, but were no longer significant following FDR correction. The MTR rs1805087 G allele and SHMT1 rs1979277 A allele were associated with higher %InAs, while the MTHFR rs1801133 T allele was associated with higher %MMAs and lower %DMAs in models predicting skin lesion risk. Nominally significant interactions were found for three SNPs [rs1540087 and rs7109250 in FOLR1 and rs1801394 in MTRR] with water As, but were no longer significant following FDR correction. Pierce et al. genotyped 19,992 variants in 4,939 Bangladeshi individuals. One SNP, rs61735836, which is located in the FTCD gene encoding formimidoyltransferase cyclodeaminase, a bifunctional enzyme that channels one-carbon units from formiminoglutamate to the folate pool, was associated with increased urinary %InAs, increased %MMAs, and decreased %DMAs, and the A allele was associated with an increased risk of skin lesions.191
Other groups have also investigated the association between OCM-related variants and As methylation efficiency. Consistent with our findings, Lindberg et al. found that the MTHFR rs1801133 T allele was associated with higher urinary %MMAs and lower %DMAs in Central Europeans (N=415).95 Although, SNPs in MTHFR were not associated with As methylation in a study of Argentinian women (N=104), among individuals with the CC (AlaAla) genotype, rs9001 located in CHDH (encoding choline dehydrogenase) was negatively significantly associated with urinary %MMAs and positively with %DMAs.192 In a case-control study of lung cancer in Argentina (N=142), Smith’s group also evaluated the association between SNPs in six OCM-related genes and As methylation, finding a positive significant association rs4920037 in cystathionine-β-synthase (CBS) and urinary %MMAs.193 Additionally, in the SHS a polymorphism in MTR was associated with higher urinary %MMA. Furthermore, a cross-sectional study conducted in Mexican women (N=1,027) found a significant interaction between folate hydrolase 1 (FOLH1) polymorphisms and vitamin B12 intake; compared to carriers of wild-type TT, CT and CC carriers had significantly lower %InAs, and higher DMA/InAs with higher vitamin B12 intake.194 In addition, a study conducted in Colombia (N=151) found significant gene-gene (AS3MT-GSTM1), gene-demographic factors (AS3MT-age) and gene-habit interactions (AS3MT-alcohol consumption) in models predicting %MMA.195 The results of these studies suggest that As methylation may be influenced by gene x nutrient x environment interactions.
2.4. One-Carbon Metabolism and Arsenic-related Health Outcomes
Observational studies have identified associations between OCM nutrients and As-related health outcomes, which may be mediated by effects on As methylation. Here we summarize research related to OCM nutrients, skin lesions, cancer, and diabetes.
2.4.1. Skin Lesions
In 2004, Smith’s group reported that, in a nested case-control study of As-related skin lesions (keratosis and melanosis) in West Bengal, India (192 cases and 192 controls), dietary folate intake was inversely associated with risk for skin lesions.196 Similarly, in a nested case-control study of skin lesions (N=274 cases and 274 controls) of Bangladeshi adults, our group found that low folate, high Hcy (hyperhomocysteinemia), and low urinary creatinine were associated with an increased risk of skin lesions.130 We later conducted a larger nested case-control study (N=876 cases and 876 controls) of As-related skin lesions, and also found that participants with hyperhomocysteinemia and lower urinary %DMAs had an increased risk of developing skin lesions within the following 2–7 years.82
OCM may have a potential role in As-related toxicity that is independent of As methylation. In our GENI study of As-related skin lesions, individuals exposed to water As exposure >50 μg/L and having a polymorphism in thymidylate synthetase (TYMS) (rs1001761) had an increased risk of developing skin lesions.82 TYMS is critical for DNA synthesis and repair and requires 5,10-methylene-THF to methylate 2-deoxy-uridine-5-monophosphate (dUMP) to form 2-deoxy-thymidine-5-monophosphate (dTMP) (Figure 4).197 Thus, we hypothesize that at higher As concentrations, TYMS-related DNA damage may be a potential mechanism of As toxicity. A study from the Stover group found that As trioxide at 0.5 μM (equivalent to 75 μg/L As in water) targets thymidylate biosynthesis.102 In this study, cell cultures exposed to As had reduced folate-dependent dTMP biosynthesis, with resulting uracil misincorporation into DNA and genomic instability. The effect of As on uracil misincorporation and genomic instability was further exacerbated by folate deficiency, revealing another potential mechanism involving folate deficiency and increased risk of developing adverse health outcomes.102
2.4.2. Cancer
Folate status was associated with urothelial carcinoma risk in a case–control study (N=177 cases and 488 controls) conducted in Taiwan of a population exposed to low water As concentrations. Higher urinary %DMAs and plasma folate concentrations were associated with a decreased risk for urothelial carcinoma. Additionally, an interaction between urinary As methylation patterns and plasma folate was observed in relation to urothelial carcinoma risk.83 In a population-based case-control study of US adults (N=1213 cases and 1418 controls), individuals with high cumulative lifetime As exposure (>22.42 mg) and insufficient folate intake (≤400 μg/day) had a higher bladder cancer risk compared to participants with both low cumulative As (≤3.52 mg) and sufficient folate intake (>400 μg/day). However, among participants with sufficient folate intake there was no association with high cumulative As exposure and bladder cancer risk.198
Arsenic and OCM-related nutrients have been shown to be associated with posttranslational histone modifications (PTHMs). An analysis of three PTHMs that are dysregulated in cancers (H3K36me2, H3K36me3, H3K79me2) was conducted within FACT (N=324) using histones isolated from PBMCs. The study found As exposure to be associated with higher levels of %H3K36me2 among men and levels of this mark decreased over time following use of As-removal filters. We also found sex-related associations between OCM-related nutrients and PTHMs. Among male participants, plasma choline was positively associated with %H3K36me2 (FDR < 0.05), whereas among female participants, plasma vitamin B12 was positively associated with %H3K79me2 (FDR < 0.01). The study also found that FA supplementation did not change the PTHMs assessed. This study suggests that OCM and PTHMs may play a role in the sex-specific risks for As-related cancer outcomes.199
2.4.3. Diabetes and Metabolic Syndromes
The SHS has identified inverse associations between urinary %MMAs and the incidence of diabetes and diabetes-related outcomes.200 In an expansion of the SHS (N=1,838) that includes family members of SHS participants known as the Strong Heart Family Study (SHFS), As exposure was associated with a higher incidence of diabetes, although exposure levels were different across regions and findings stratified by regions were not reported. Additionally, in a pilot study within a subset (N=59) of the SHFS), waist circumference was inversely associated with %MMA and positively associated with %DMA in urine. However, adjustment for OCM-related nutrients, including glutamate and choline-related metabolites, attenuated and reversed the direction of these associations.201 BMI has also been associated with As species in female but not in male participants in FACT (N=527) and were similar but not significant in FOX (N=342). The adjusted mean differences (95% CI) in female participants’ urinary MMA% and DMA% for a 5 kg/m2 difference in BMI were −1.21 (−1.96, −0.45) and 2.47 (1.13, 3.81), respectively in FACT, and −0.66 (−1.56, 0.25) and 1.43 (−0.23, 3.09) in FOX. However, these associations were null after adjustment for choline suggesting that choline is an important confounder of the association between BMI and As methylation.96 The observed association between lower urinary %MMA and higher %DMA with diabetes- and obesity-related outcomes may be influenced by OCM status and further research is required to assess whether these associations are causally related.201
Additionally, in the SHS, a polymorphism in MTR was associated with higher urinary %MMA and lower insulin resistance as measured by Homeostatic Model Assessment Index (HOMA2-IR). While adjustment for OCM variables (SNPs in: PEMT, MTR, MTRR, MTHFR, MTHFD1, SHMT1 and CBS; and nutrient intake: vitamin B6, vitamin B2, and folate), did not affect the associations between As methylation and diabetes-related outcomes, including HOMA2-IR, risk for diabetes, and metabolic syndrome, the association between the MTR variant and HOMA2-IR was no longer significant after adjustment for urinary %MMAs.202
A study conducted in the New Hampshire Birth Cohort (N=1,151) found a significant positive association between maternal toenail As concentrations and GDM.53 Mothers with GDM have been shown to have a 7-fold increased risk of developing Type II diabetes 10 years after pregnancy, and infants born to mothers with GDM have an increased risk of developing glucose intolerance and cardiometabolic disease in the future.203–206 A review conducted by members of our group evaluated the influence of maternal nutritional status, including folate, on the association between early-life As exposure and risk of diabetes and diabetes-related outcomes in experimental and epidemiological studies. The review found that in four out of five experimental studies, rodent offspring exposed to As in utero had elevated insulin resistance, with evidence suggesting maternal supplementation with folate + B12 was protective against As-induced hyperglycemia in the offspring.185 Additionally, increased in utero As exposure was related to changes in cord blood gene expression, microRNA and DNA methylation profiles involved in diabetes-related pathways among birth cohorts of As-exposed mothers residing in New Hampshire, Mexico, and Taiwan. The review highlighted a gap in the research regarding the evaluation of prevention strategies such as nutritional interventions that can modify As-related disease risk.207
2.5. Mathematical Models of One-Carbon Metabolism and Arsenic Methylation
To aid in interpretation of the data from our clinical trials in Bangladesh on the effects of FA and/or creatine supplementation on As methylation and excretion, our collaborators, Michael C. Reed and H. Frederik Nijhout at Duke University, developed a whole-body mathematical model of As methylation that includes absorption, storage, methylation, and excretion. Parameters for hepatic As methylation were based on biochemical literature, and compartments for the binding of arsenicals to intracellular proteins were included for model fit. Parameters for transport of As between tissues were adjusted to accurately predict the As urine excretion rates in single-dose experiments of human subjects. The model was able to reproduce decreases in blood As observed in our first clinical trial of folate-deficient individuals, and predicted that As concentrations would decrease by 19% in the liver and 26% other body stores.208
These mathematical models were further used to better understand the modest changes in As methylation observed with creatine supplementation in FACT.209 Our research question was to determine if synthesis of creatine by guanidinoacetate-N-methyltransferase decreased, how much would hepatic SAM increase, and consequently, how much would that increase the activity of AS3MT. When one methyltransferase enzyme is downregulated, there are several long-range allosteric interactions that buffer the flux through other methyltransferases. Therefore, in order to increase As methylation with creatine supplementation, at least one or more of these regulatory mechanisms would need to be disrupted. Because glycine-N-methyltransferase (GNMT) regulates hepatic SAM concentrations and is inhibited by binding to 5-methyl-THF, increasing hepatic 5-methyl-THF concentrations should allow creatine supplementation to increase SAM by inhibiting GNMT. Even in folate sufficient individuals, FA supplementation substantially increases plasma and RBC folate concentrations, but it is unknown to what extent it increases liver folate and liver SAM in replete individuals. Thus, the mathematical models of OCM have helped us to better understand why the effects of creatine supplementation on As methylation, while statistically significant, were modest.163 Furthermore, these models have the potential to inform future nutritional interventions.
3. Conclusions and Future Directions
Chronic exposure to As is a global public health concern associated with many adverse health outcomes, including skin lesions, CVD, diabetes and diabetes-related outcomes, and various cancers. Urinary As excretion is facilitated by As methylation in a process that is dependent on OCM, which involves many micronutrients including folate, vitamin B12, choline, betaine, vitamin B6, riboflavin, niacin and creatine. Particularly among populations living in regions without FA-fortification programs, epidemiological studies and RCTs have provided strong evidence that folate status and FA supplementation increase As methylation capacity, thereby facilitating As elimination and lowering blood As concentrations. However, there is a lack of information regarding the role of other OCM-related micronutrients in As methylation, elimination, and toxicity. A major gap in knowledge is whether the association between OCM, As methylation, and metabolic-related health outcomes such as diabetes are a result of confounding, mediation, or reverse causality. Additionally, there is a lack of definitive evidence that nutritional interventions such as FA supplementation can reduce the risk of As-related health outcomes. There is some evidence from nested case-control studies that better folate status is associated with reduced risk for As-related skin lesions130,196 and bladder cancer.198 However, the protective effects of FA for other As-related adverse health outcomes have not been established and will require further research. Innovative study designs may be needed because, while RCTs may be considered the gold standard for providing definitive answers to these critical remaining research questions, new proposals to conduct large scale, long term FA RCTs may raise ethical concerns, as there are clear benefits of FA supplementation on As methylation efficiency.
Mandatory food FA-fortification programs currently exist in more than 80 countries worldwide. Folate fortification has been shown to nearly eradicate folate deficiency and the associated adverse health outcomes in Western countries such as the United States and Canada. However, mandatory FA-fortification programs do not exist in most countries having widespread exposure to As-contaminated drinking water. While the highest priority should be to provide access to safe drinking water, FA-fortification programs in As-endemic countries may help to eliminate folate deficiency with further benefits of aiding in a reduction of the public health consequences of As exposure.
ACKNOWLEDGMENTS
We thank Michael C. Reed, Department of Mathematics, Duke University, and H. Frederik Nijhout, Department of Biology, Duke University, who have collaborated in modeling OCM.
Abbreviations
- As
Arsenic
- AS3MT
arsenic-3-methyltransferase
- BHMT
betaine homocysteine methyltransferase
- BHMT
betaine-homocysteine S-methyltransferase
- CBS
cystathionine-β-synthase
- CHDH
choline dehydrogenase
- CVD
cardiovascular disease
- DMAs
dimethyl arsenical species
- DMAsV
dimethylarsinic acid
- DNMT3A
DNA methyltransferase 3 alpha
- dTMP
2-deoxy-thymidine-5-monophosphate
- dUMP
2-deoxy-uridine-5-monophosphate
- EPA
Environmental Protection Agency
- FA
folic acid
- FACT
Folic Acid and Creatine Trial
- FDA
Food and Drug Administration
- FDR
false discovery rate
- Folbp
folate-binding protein
- FOLH1
folate hydrolase 1
- FOLR1
folate receptor 1
- FOLR2
folate receptor 2
- FOX
Folate and Oxidative Stress
- FTCD
formimidoyltransferase cyclodeaminase
- GAA
guanidinoacetate
- GAMT
guanidinoacetate methyltransferase
- GDM
gestational diabetes mellitus
- GENI
Gene Environment Nutrient Interactions
- GM
gut microbiome
- GNMT
glycine-N-methyltransferase
- GSH
glutathione
- Hcy
homocysteine
- HEALS
Health Effects of Arsenic Longitudinal Study
- HOMA2-IR
Homeostatic Model Assessment Index
- InAs
inorganic arsenic
- InAsIII
arsenite
- InAsV
arsenate
- LOD
limit of detection
- MMAs
Monomethyl arsenic species
- MMAsIII
monomethylarsonous acid
- MMAsV
monomethylarsonic acid
- MTHFR
methylene tetrahydrofolate reductase
- MTR
methionine synthase
- MTRR
methionine synthase reductase
- NAD
nicotinamide adenine dinucleotide
- NHANES
National Health and Nutrition Examination Survey
- NTDs
neural tube defects
- OCM
one-carbon metabolism
- PBMC
peripheral blood mononuclear cell
- PC
phosphatidyl choline
- PEMT
phosphatidylethanolamine methyltransferase
- PTHMs
posttranslational histone modifications
- RBC folate
red blood cell folate
- RCT
randomized control trial
- ROS
reactive oxygen species
- SAH
S-adenosylhomocysteine
- SAM
S-adenosylmethionine
- SHMT
serine hydroxymethyltransferase
- SHMT1
serine hydroxymethyltransferase 1
- SHS
Strong Heart Study
- SHFS
Strong Heart Family Study
- THF
tetrahydrofolate
- TMAO
tri-methylamine oxide
- TYMS
thymidylate synthetase
- US
United States
- UNICEF
United Nations Children’s Fund
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bozack AK, Saxena R. & Gamble MV Nutritional Influences on One-Carbon Metabolism: Effects on Arsenic Methylation and Toxicity. Annual Review of Nutrition 38, 401–429, doi: 10.1146/annurev-nutr-082117-051757 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Darnton-Hill I. Fortification of wheat flour: Biological, behavioural and contextual rationale (World Health Organization (WHO), University of Sydney, Australia, 2017). [Google Scholar]
- 3.Welch AH & Stollenwerk KG in Arsenic in groundwater: Geochemistry and occurence (Kluwer Academic Publishers, New York, 2002). [Google Scholar]
- 4.World Health Organization (WHO). Guidelines for drinking-water quality: Incorporating first and second addenda to third edition. (Geneva, Switzerland, 2008). [PubMed] [Google Scholar]
- 5.Environmental Protection Agency (EPA). Technical Fact Sheet: Final Rule for Arsenic in Drinking Water. Report No. EPA-815-F-00–016, (2001). [Google Scholar]
- 6.Ravenscroft P, Brammer H. & Richards K. Arsenic pollution: A global synthesis. (Wiley-Blackwell, 2009). [Google Scholar]
- 7.Smith AH, Lingas EO & Rahman M. Contamination of drinking-water by arsenic in Bangladesh: A public health emergency. Bulletin of the World Health Organization 78, 1093–1103 (2000). [PMC free article] [PubMed] [Google Scholar]
- 8.Ayotte JD, Ann Gronberg JM & Apodaca L. National water-quality assessment program trace elements and radon in groundwater across the United States. (Reston, Virginia, 2011). [Google Scholar]
- 9.Nigra AE et al. Inequalities in Public Water Arsenic Concentrations in Counties and Community Water Systems across the United States, 2006–2011. Environmental Health Perspectives 128, 127001, doi: 10.1289/EHP7313 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayotte JD, Medalie L, Qi SL, Backer LC & Nolan BT Estimating the High-Arsenic Domestic-Well Population in the Conterminous United States. Environmental Science & Technology 51, 12443–12454, doi: 10.1021/acs.est.7b02881 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Environmental Protection Agency (EPA). National primary drinking water regulations; arsenic and clarifications to compliance and new cource contaminants: Final rule. 66:6996–706. (2001). [Google Scholar]
- 12.He Y. & Zheng Y. Assessment of in vivo bioaccessibility of arsenic in dietary rice by a mass balance approach. Science of The Total Environment 408, 1430–1436, doi: 10.1016/j.scitotenv.2009.12.043 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meharg AA et al. Urinary excretion of arsenic following rice consumption. Environmental Pollution 194, 181–187, doi: 10.1016/j.envpol.2014.07.031 (2014). [DOI] [PubMed] [Google Scholar]
- 14.deCastro BR et al. Dietary Sources of Methylated Arsenic Species in Urine of the United States Population, NHANES 2003–2010. PLOS ONE 9, e108098, doi: 10.1371/journal.pone.0108098 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilbert-Diamond D. et al. Rice consumption contributes to arsenic exposure in US women. Proceedings of the National Academy of Sciences of the United States of America 108, 20656–20660 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams PN et al. Variation in arsenic speciation and concentration in paddy rice related to dietary exposure. Environmental Science & Technology 39, 5531–5540, doi: 10.1021/es0502324 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Food & Drug Administration (FDA). Arsenic in rice and rice products: Risk assessment report. (2016). [Google Scholar]
- 18.Zhang R. et al. Rice consumption and cancer incidence in US men and women. International Journal of Cancer 138, 555–564, doi: 10.1002/ijc.29704 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muraki I. et al. Rice consumption and risk of cardiovascular disease: results from a pooled analysis of 3 U.S. cohorts. The American Journal of Clinical Nutrition 101, 164–172, doi: 10.3945/ajcn.114.087551 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eshak ES et al. Rice consumption is not associated with risk of cardiovascular disease morbidity or mortality in Japanese men and women: a large population-based, prospective cohort study. The American Journal of Clinical Nutrition 100, 199–207, doi: 10.3945/ajcn.113.079038 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Wilson D, Hooper C. & Shi X. Arsenic and lead in juice: Apple, citrus, and apple-base. Journal of Environmental Health 75, 14–20; quiz 44 (2012). [PubMed] [Google Scholar]
- 22.Wilson D. Arsenic Content in American Wine. J Environ Health 78, 16–22 (2015). [PubMed] [Google Scholar]
- 23.Hall M. et al. Determinants of arsenic metabolism: Blood arsenic metabolites, plasma folate, cobalamin, and homocysteine concentrations in maternal-newborn pairs. Environmental Health Perspectives 115, 1503–1509, doi: 10.1289/ehp.9906 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Concha G, Vogler G, Lezcano D, Nermell B. & Vahter M. Exposure to inorganic arsenic metabolites during early human development. Toxicological Sciences 44, 185–190, doi: 10.1006/TOXS.1998.2486 (1998). [DOI] [PubMed] [Google Scholar]
- 25.Concha G, Vogler G, Nermell B. & Vahter M. Low-level arsenic excretion in breast milk of native Andean women exposed to high levels of arsenic in the drinking water. International Archives of Occupational and Environmental Health 71, 42–46, doi: 10.1007/s004200050248 (1998). [DOI] [PubMed] [Google Scholar]
- 26.Fängström B. et al. Breast-feeding Protects against Arsenic Exposure in Bangladeshi Infants. Environmental Health Perspectives 116, 963–969, doi: 10.1289/ehp.11094 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nriagu J. & Azcue J. Arsenic in the environment. Part I. Cycling and characterization. 1–15 (Wiley, 1990). [Google Scholar]
- 28.Pal A. et al. Additional danger of arsenic exposure through inhalation from burning of cow dung cakes laced with arsenic as a fuel in arsenic affected villages in Ganga–Meghna–Brahmaputra plain. Journal of Environmental Monitoring 9, 1067–1070, doi: 10.1039/B709339J (2007). [DOI] [PubMed] [Google Scholar]
- 29.American Cancer Society. Known and probable human carcinogens, <https://www.cancer.org/cancer/cancer-causes/general-info/known-and-probable-human-carcinogens.html> (2016).
- 30.Grau-Perez M. et al. Association of Low-Moderate Arsenic Exposure and Arsenic Metabolism with Incident Diabetes and Insulin Resistance in the Strong Heart Family Study. Environmental Health Perspectives 125, 127004–127004, doi: 10.1289/EHP2566 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naujokas MF et al. The broad scope of health effects from chronic arsenic exposure: Update on a worldwide public health problem. Environmental Health Perspectives 121, 295 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saba S. et al. Assessment of heavy metals by ICP-OES and their impact on insulin stimulating hormone and carbohydrate metabolizing enzymes. Clinical and Experimental Pharmacology and Physiology n/a, doi: 10.1111/1440-1681.13353 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Kazemifar AM, Shafikhani AA, Mozhdehipanah H, Khamesi S. & Arami M. Evaluation of different types of arsenic methylation and its relationship with metabolic syndrome in an area chronically exposed to arsenic. Environ Anal Health Toxicol 35, e2020006-e2020006, doi: 10.5620/eaht.e2020006 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith AH et al. Lung, Bladder, and Kidney Cancer Mortality 40 Years After Arsenic Exposure Reduction. JNCI: Journal of the National Cancer Institute 110, 241–249, doi: 10.1093/jnci/djx201 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.López-Carrillo L, Gamboa-Loira B, Gandolfi AJ & Cebrián ME Inorganic arsenic methylation capacity and breast cancer by immunohistochemical subtypes in northern Mexican women. Environmental Research 184, 109361, doi: 10.1016/j.envres.2020.109361 (2020). [DOI] [PubMed] [Google Scholar]
- 36.García-Esquinas E. et al. Arsenic exposure and cancer mortality in a US-based prospective cohort: the Strong Heart Study. Cancer Epidemiology, Biomarkers, & Prevention 22, 1944–1953, doi: 10.1158/1055-9965.Epi-13-0234-t (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tseng WP Effects and dose-response relationships of skin cancer and blackfoot disease with arsenic. Environmental Health Perspectives 19, 109–119 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frediani JK et al. Arsenic exposure and risk of nonalcoholic fatty liver disease (NAFLD) among U.S. adolescents and adults: an association modified by race/ethnicity, NHANES 2005–2014. Environmental Health 17, 6, doi: 10.1186/s12940-017-0350-1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuo CC et al. Early-life arsenic exposure promotes atherogenic lipid metabolism in adolescence: A 15-year birth cohort follow-up study in central Taiwan. Environment International 118, 97–105, doi: 10.1016/j.envint.2018.05.033 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Liao N. et al. A Comprehensive Review of Arsenic Exposure and Risk from Rice and a Risk Assessment among a Cohort of Adolescents in Kunming, China. Int J Environ Res Public Health 15, doi: 10.3390/ijerph15102191 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y. et al. Early life and adolescent arsenic exposure from drinking water and blood pressure in adolescence. Environmental Research 178, 108681, doi: 10.1016/j.envres.2019.108681 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tseng HP et al. Association between chronic exposure to arsenic and slow nerve conduction velocity among adolescents in Taiwan. Journal of Health, Population, and Nutrition 24, 182–189 (2006). [PubMed] [Google Scholar]
- 43.Vibol S, Hashim JH & Sarmani S. Neurobehavioral effects of arsenic exposure among secondary school children in the Kandal Province, Cambodia. Environmental Research 137, 329–337, doi: 10.1016/j.envres.2014.12.001 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Wasserman GA et al. A cross-sectional study of well water arsenic and child IQ in Maine schoolchildren. Environmental Health 13, 23, doi: 10.1186/1476-069x-13-23 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rahman A. et al. Arsenic exposure and risk of spontaneous abortion, stillbirth, and infant mortality. Epidemiology 21, 797–804, doi: 10.1097/EDE.0b013e3181f56a0d (2010). [DOI] [PubMed] [Google Scholar]
- 46.Bloom MS, Surdu S, Neamtiu IA & Gurzau ES Maternal arsenic exposure and birth outcomes: a comprehensive review of the epidemiologic literature focused on drinking water. Int J Hyg Environ Health 217, 709–719, doi: 10.1016/j.ijheh.2014.03.004 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Milton AH et al. A Review of the Effects of Chronic Arsenic Exposure on Adverse Pregnancy Outcomes. International Journal of Environmental Research and Public Health 14 (2017). < 10.3390/ijerph14060556>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vahter M. Effects of Arsenic on Maternal and Fetal Health. Annual Review of Nutrition 29, 381–399, doi: 10.1146/annurev-nutr-080508-141102 (2009). [DOI] [PubMed] [Google Scholar]
- 49.Zhong Q. et al. Association of maternal arsenic exposure with birth size: A systematic review and meta-analysis. Environmental Toxicology and Pharmacology 69, 129–136, doi: 10.1016/j.etap.2019.04.007 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Raqib R. et al. Effects of in utero arsenic exposure on child immunity and morbidity in rural Bangladesh. Toxicology Letters 185, 197–202, doi: 10.1016/j.toxlet.2009.01.001 (2009). [DOI] [PubMed] [Google Scholar]
- 51.Smith AH et al. Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Environmental Health Perspectives 114, 1293–1296 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith AH et al. Mortality in young adults following in utero and childhood exposure to arsenic in drinking water. Environmental Health Perspectives 120, 1527–1531, doi: 10.1289/ehp.1104867 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farzan SF et al. Maternal arsenic exposure and gestational diabetes and glucose intolerance in the New Hampshire birth cohort study. Environmental Health 15, 106, doi: 10.1186/s12940-016-0194-0 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buchet JP, Lauwerys R. & Roels H. Comparison of the urinary excretion of arsenic metabolites after a single oral dose of sodium arsenite, monomethylarsonate, or dimethylarsinate in man. International Archives of Occupational and Environmental Health 48, 71–79, doi: 10.1007/BF00405933 (1981). [DOI] [PubMed] [Google Scholar]
- 55.Challenger F. Biological methylation. Chemical Reviews 36, 315–361 (1945). [Google Scholar]
- 56.Lin S. et al. A novel S-adenosyl-L-methionine:arsenic(III) methyltransferase from rat liver cytosol. The Journal of Biological Chemistry 277, 10795–10803, doi: 10.1074/jbc.M110246200 (2002). [DOI] [PubMed] [Google Scholar]
- 57.Thomas DJ, Waters SB & Styblo M. Elucidating the pathway for arsenic methylation. Toxicology and Applied Pharmacology 198, 319–326, doi: 10.1016/j.taap.2003.10.020 (2004). [DOI] [PubMed] [Google Scholar]
- 58.Petrick JS, Ayala-Fierro F, Cullen WR, Carter DE & Vasken Aposhian H. Monomethylarsonous acid (MMA(III)) is more toxic than arsenite in Chang human hepatocytes. Toxicology and Applied Pharmacology 163, 203–207, doi: 10.1006/taap.1999.8872 (2000). [DOI] [PubMed] [Google Scholar]
- 59.Petrick JS, Jagadish B, Mash EA & Aposhian HV Monomethylarsonous acid (MMA(III)) and arsenite: LD(50) in hamsters and in vitro inhibition of pyruvate dehydrogenase. Chemical Research in Toxicology 14, 651–656 (2001). [DOI] [PubMed] [Google Scholar]
- 60.Styblo M. et al. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Archives of Toxicology 74, 289–299 (2000). [DOI] [PubMed] [Google Scholar]
- 61.Drobna Z. et al. Disruption of the arsenic (+3 oxidation state) methyltransferase gene in the mouse alters the phenotype for methylation of arsenic and affects distribution and retention of orally administered arsenate. Chemical Research in Toxicology 22, 1713–1720, doi: 10.1021/tx900179r (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hughes MF et al. Arsenic (+3 oxidation state) methyltransferase genotype affects steady-state distribution and clearance of arsenic in arsenate-treated mice. Toxicology and Applied Pharmacology 249, 217–223, doi: 10.1016/j.taap.2010.09.017 (2010). [DOI] [PubMed] [Google Scholar]
- 63.Gamble MV et al. Folic acid supplementation lowers blood arsenic. The American Journal of Clinical Nutrition 86, 1202–1209 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kalman DA et al. Occurrence of trivalent monomethyl arsenic and other urinary arsenic species in a highly exposed juvenile population in Bangladesh. J Expo Sci Environ Epidemiol 24, 113–120, doi: 10.1038/jes.2013.14 (2014). [DOI] [PubMed] [Google Scholar]
- 65.Moe B. et al. Comparative cytotoxicity of fourteen trivalent and pentavalent arsenic species determined using real-time cell sensing. Journal of Environmental Sciences (China) 49, 113–124, doi: 10.1016/j.jes.2016.10.004 (2016). [DOI] [PubMed] [Google Scholar]
- 66.Gamboa-Loira B, Cebrián ME, Franco-Marina F. & López-Carrillo L. Arsenic metabolism and cancer risk: A meta-analysis. Environmental Research 156, 551–558, doi: 10.1016/j.envres.2017.04.016 (2017). [DOI] [PubMed] [Google Scholar]
- 67.Steinmaus C. et al. Arsenic methylation and bladder cancer risk in case-control studies in Argentina and the United States. Journal of Occupational and Environmental Medicine 48, 478–488, doi: 10.1097/01.jom.0000200982.28276.70 (2006). [DOI] [PubMed] [Google Scholar]
- 68.Pu Y-S et al. Urinary arsenic profile affects the risk of urothelial carcinoma even at low arsenic exposure. Toxicology and Applied Pharmacology 218, 99–106, doi: 10.1016/j.taap.2006.09.021 (2007). [DOI] [PubMed] [Google Scholar]
- 69.Melak D. et al. Arsenic methylation and lung and bladder cancer in a case-control study in northern Chile. Toxicology and Applied Pharmacology 274, 225–231, doi: 10.1016/j.taap.2013.11.014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chung C-J et al. Urinary arsenic profiles and the risks of cancer mortality: A population-based 20-year follow-up study in arseniasis-endemic areas in Taiwan. Environmental Research 122, 25–30, doi: 10.1016/j.envres.2012.11.007 (2013). [DOI] [PubMed] [Google Scholar]
- 71.Lopez-Carrillo L. et al. Arsenic methylation capacity is associated with breast cancer in northern Mexico. Toxicology and Applied Pharmacology 280, 53–59, doi: 10.1016/j.taap.2014.07.013 (2014). [DOI] [PubMed] [Google Scholar]
- 72.Steinmaus C. et al. Individual differences in arsenic metabolism and lung cancer in a case-control study in Cordoba, Argentina. Toxicology and Applied Pharmacology 247, 138–145, doi: 10.1016/j.taap.2010.06.006 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gilbert-Diamond D. et al. A population-based case-control study of urinary arsenic species and squamous cell carcinoma in New Hampshire, USA. Environmental Health Perspectives 121, 1154–1160, doi: 10.1289/ehp.1206178 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu RC, Hsu KH, Chen CJ & Froines JR Arsenic methylation capacity and skin cancer. Cancer Epidemiology, Biomarkers & Prevention 9, 1259–1262 (2000). [PubMed] [Google Scholar]
- 75.Ahsan H. et al. Arsenic metabolism, genetic susceptibility, and risk of premalignant skin lesions in Bangladesh. Cancer Epidemiology, Biomarkers & Prevention 16, 1270–1278, doi: 10.1158/1055-9965.epi-06-0676 (2007). [DOI] [PubMed] [Google Scholar]
- 76.Lindberg A-L, Rahman M, Persson L-A & Vahter M. The risk of arsenic induced skin lesions in Bangladeshi men and women is affected by arsenic metabolism and the age at first exposure. Toxicology and Applied Pharmacology 230, 9–16, doi: 10.1016/j.taap.2008.02.001 (2008). [DOI] [PubMed] [Google Scholar]
- 77.Zhang Q. et al. Joint effects of urinary arsenic methylation capacity with potential modifiers on arsenicosis: A cross-sectional study from an endemic arsenism area in Huhhot Basin, northern China. Environmental Research 132, 281–289, doi: 10.1016/j.envres.2014.04.036 (2014). [DOI] [PubMed] [Google Scholar]
- 78.Wu M-M et al. Effect of plasma homocysteine level and urinary monomethylarsonic acid on the risk of arsenic-associated carotid atherosclerosis. Toxicology and Applied Pharmacology 216, 168–175, doi: 10.1016/j.taap.2006.05.005 (2006). [DOI] [PubMed] [Google Scholar]
- 79.Tseng CH et al. Arsenic exposure, urinary arsenic speciation, and peripheral vascular disease in blackfoot disease-hyperendemic villages in Taiwan. Toxicol Appl Pharmacol 206, 299–308, doi: 10.1016/j.taap.2004.11.022 (2005). [DOI] [PubMed] [Google Scholar]
- 80.Newman JD et al. Peripheral Arterial Disease and Its Association With Arsenic Exposure and Metabolism in the Strong Heart Study. American Journal of Epidemiology 184, 806–817, doi: 10.1093/aje/kww002 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen Y-C et al. Arsenic methylation and bladder cancer risk in Taiwan. Cancer Causes & Control 14, 303–310 (2003b). [DOI] [PubMed] [Google Scholar]
- 82.Niedzwiecki MM et al. Serum homocysteine, arsenic methylation, and arsenic-induced skin lesion incidence in Bangladesh: A one-carbon metabolism candidate gene study. Environment International 113, 133–142, doi: 10.1016/j.envint.2018.01.015 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang YK et al. Plasma folate level, urinary arsenic methylation profiles, and urothelial carcinoma susceptibility. Food and Chemical Toxicology 46, 929–938, doi: 10.1016/j.fct.2007.10.017 (2008). [DOI] [PubMed] [Google Scholar]
- 84.Huang Y-K et al. Arsenic methylation capability and hypertension risk in subjects living in arseniasis-hyperendemic areas in southwestern Taiwan. Toxicology and Applied Pharmacology 218, 135–142, doi: 10.1016/j.taap.2006.10.022 (2007). [DOI] [PubMed] [Google Scholar]
- 85.Li X. et al. Prolonged environmental exposure of arsenic through drinking water on the risk of hypertension and type 2 diabetes. Environmental Science and Pollution Research International 20, 8151–8161, doi: 10.1007/s11356-013-1768-9 (2013a). [DOI] [PubMed] [Google Scholar]
- 86.Li X. et al. Association of urinary monomethylated arsenic concentration and risk of hypertension: A cross-sectional study from arsenic contaminated areas in northwestern China. Environmental Health 12, 37, doi: 10.1186/1476-069x-12-37 (2013b). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li Y, Wang D, Li X, Zheng Q. & Sun G. A potential synergy between incomplete arsenic methylation capacity and demographic characteristics on the risk of hypertension: Findings from a cross-sectional study in an arsenic-endemic area of inner Mongolia, China. International Journal of Environmental Research and Public Health 12, 3615–3632, doi: 10.3390/ijerph120403615 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kuo CC et al. Arsenic Exposure, Arsenic Metabolism, and Incident Diabetes in the Strong Heart Study. Diabetes Care 38, 620–627, doi: 10.2337/dc14-1641 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Q. et al. Interactions of arsenic metabolism with arsenic exposure and individual factors on diabetes occurrence: Baseline findings from Arsenic and Non-Communicable disease cohort (AsNCD) in China. Environmental Pollution 265, 114968, doi: 10.1016/j.envpol.2020.114968 (2020). [DOI] [PubMed] [Google Scholar]
- 90.Spratlen MJ et al. The Association of Arsenic Exposure and Arsenic Metabolism With the Metabolic Syndrome and Its Individual Components: Prospective Evidence From the Strong Heart Family Study. American Journal of Epidemiology 187, 1598–1612, doi: 10.1093/aje/kwy048 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen J-W et al. Arsenic methylation, GSTO1 polymorphisms, and metabolic syndrome in an arseniasis endemic area of southwestern Taiwan. Chemosphere 88, 432–438, doi: 10.1016/j.chemosphere.2012.02.059 (2012). [DOI] [PubMed] [Google Scholar]
- 92.Mendez MA et al. Chronic Exposure to Arsenic and Markers of Cardiometabolic Risk: A Cross-Sectional Study in Chihuahua, Mexico. Environmental Health Perspectives 124, 104–111, doi: 10.1289/ehp.1408742 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gomez-Rubio P. et al. Association between body mass index and arsenic methylation efficiency in adult women from southwest U.S. and northwest Mexico. Toxicol Appl Pharmacol 252, 176–182, doi: 10.1016/j.taap.2011.02.007 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bommarito PA et al. One-carbon metabolism nutrient intake and the association between body mass index and urinary arsenic metabolites in adults in the Chihuahua cohort. Environment International 123, 292–300, doi: 10.1016/j.envint.2018.12.004 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lindberg A-L et al. Metabolism of low-dose inorganic arsenic in a central European population: influence of sex and genetic polymorphisms. Environmental Health Perspectives 115, 1081–1086, doi: 10.1289/ehp.10026 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abuawad A. et al. Association between body mass index and arsenic methylation in three studies of Bangladeshi adults and adolescents. Environment International 149, 106401, doi: 10.1016/j.envint.2021.106401 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gao J. et al. The genetic architecture of arsenic metabolism efficiency: A SNP-based heritability study of Bangladeshi adults. Environmental Health Perspectives 123, 985–992, doi: 10.1289/ehp.1408909 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jansen RJ et al. Determinants and Consequences of Arsenic Metabolism Efficiency among 4,794 Individuals: Demographics, Lifestyle, Genetics, and Toxicity. Cancer Epidemiology Biomarkers & Prevention 25, 381, doi: 10.1158/1055-9965.EPI-15-0718 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gomez-Rubio P, Klimecki W, Meza-Montenegro M. & Cantu-Soto E. Genetic association between intronic variants in AS3MT and arsenic methylation efficiency is focused on a large linkage disequilibrium cluster in chromosome 10. J Appl Toxicol 30 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Engström KS et al. Genetic variation in arsenic (+3 oxidation state) methyltransferase (AS3MT), arsenic metabolism and risk of basal cell carcinoma in a European population. Environmental and Molecular Mutagenesis 56, 60–69, doi: 10.1002/em.21896 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gosse JA, Taylor VF, Jackson BP, Hamilton JW & Bodwell JE Monomethylated trivalent arsenic species disrupt steroid receptor interactions with their DNA response elements at non-cytotoxic cellular concentrations. Journal of Applied Toxicology 34, 498–505, doi: 10.1002/jat.2898 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kamynina E. et al. Arsenic trioxide targets MTHFD1 and SUMO-dependent nuclear de novo thymidylate biosynthesis. Proceedings of the National Academy of Sciences of the United States of America 114, E2319-e2326, doi: 10.1073/pnas.1619745114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Muenyi CS, Ljungman M. & States JC Arsenic disruption of DNA damage responses-potential role in carcinogenesis and chemotherapy. Biomolecules 5, 2184–2193, doi: 10.3390/biom5042184 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pelch KE, Tokar EJ, Merrick BA & Waalkes MP Differential DNA methylation profile of key genes in malignant prostate epithelial cells transformed by inorganic arsenic or cadmium. Toxicology and Applied Pharmacology 286, 159–167, doi: 10.1016/j.taap.2015.04.011 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reichard JF & Puga A. Effects of arsenic exposure on DNA methylation and epigenetic gene regulation. Epigenomics 2, 87–104, doi: 10.2217/epi.09.45 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu Y, Tokar EJ & Waalkes MP Arsenic-induced cancer cell phenotype in human breast epithelia is estrogen receptor-independent but involves aromatase activation. Archives of Toxicology 88, 263–274, doi: 10.1007/s00204-013-1131-4 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Singh AP, Goel RK & Kaur T. Mechanisms pertaining to arsenic toxicity. Toxicology International 18, 87–93, doi: 10.4103/0971-6580.84258 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chi L. et al. Gut microbiome disruption altered the biotransformation and liver toxicity of arsenic in mice. Archives of Toxicology 93, 25–35, doi: 10.1007/s00204-018-2332-7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Coryell M, McAlpine M, Pinkham NV, McDermott TR & Walk ST The gut microbiome is required for full protection against acute arsenic toxicity in mouse models. Nature Communications 9, 5424, doi: 10.1038/s41467-018-07803-9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yokohira M. et al. Severe systemic toxicity and urinary bladder cytotoxicity and regenerative hyperplasia induced by arsenite in arsenic (+3 oxidation state) methyltransferase knockout mice. A preliminary report. Toxicology and Applied Pharmacology 246, 1–7, doi: 10.1016/j.taap.2010.04.013 (2010). [DOI] [PubMed] [Google Scholar]
- 111.Lu K. et al. Gut Microbiome Perturbations Induced by Bacterial Infection Affect Arsenic Biotransformation. Chemical Research in Toxicology 26, 1893–1903, doi: 10.1021/tx4002868 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lu K. et al. Gut Microbiome Phenotypes Driven by Host Genetics Affect Arsenic Metabolism. Chemical Research in Toxicology 27, 172–174, doi: 10.1021/tx400454z (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Steinmaus C. et al. Increased lung and bladder cancer incidence in adults after in utero and early-life arsenic exposure. Cancer Epidemiology, Biomarkers & Prevention 23, 1529–1538, doi: 10.1158/1055-9965.epi-14-0059 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Steinmaus CM et al. Drinking water arsenic in Northern Chile: High cancer risks 40 years after exposure cessation. Cancer Epidemiology, Biomarkers & Prevention 22, 623–630, doi: 10.1158/1055-9965.epi-12-1190 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cui X, Wakai T, Shirai Y, Hatakeyama K. & Hirano S. Chronic Oral Exposure to Inorganic Arsenate Interferes with Methylation Status of p16INK4a and RASSF1A and Induces Lung Cancer in A/J Mice. Toxicological Sciences 91, 372–381, doi: 10.1093/toxsci/kfj159 (2006). [DOI] [PubMed] [Google Scholar]
- 116.van Breda SGJ et al. Epigenetic mechanisms underlying arsenic-associated lung carcinogenesis. Archives of Toxicology 89, 1959–1969, doi: 10.1007/s00204-014-1351-2 (2015). [DOI] [PubMed] [Google Scholar]
- 117.Argos M. Arsenic Exposure and Epigenetic Alterations: Recent Findings Based on the Illumina 450K DNA Methylation Array. Curr Environ Health Rep 2, 137–144, doi: 10.1007/s40572-015-0052-1 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Howe CG & Gamble MV Influence of arsenic on global levels of histone posttranslational modifications: a review of the literature and challenges in the field. Current Environmental Health Reports 3, 225–237, doi: 10.1007/s40572-016-0104-1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mauro M, Caradonna F, Klein CB & Dolinoy D. Dysregulation of DNA methylation induced by past arsenic treatment causes persistent genomic instability in mammalian cells. Environmental and Molecular Mutagenesis 57, 137–150, doi: 10.1002/em.21987 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Niedzwiecki MM et al. A dose-response study of arsenic exposure and global methylation of peripheral blood mononuclear cell DNA in Bangladeshi adults. Environmental Health Perspectives 121, 1306, doi: 10.1289/ehp.1206421 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pilsner JR et al. Genomic methylation of peripheral blood leukocyte DNA: Influences of arsenic and folate in Bangladeshi adults. The American Journal of Clinical Nutrition 86, 1179–1186 (2007). [DOI] [PubMed] [Google Scholar]
- 122.Pilsner JR et al. Influence of prenatal arsenic exposure and newborn sex on global methylation of cord blood DNA. PLoS One 7, e37147, doi: 10.1371/journal.pone.0037147 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kile ML et al. Prenatal arsenic exposure and DNA methylation in maternal and umbilical cord blood leukocytes. Environmental Health Perspectives 120, 1061–1066, doi: 10.1289/ehp.1104173 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Argos M. et al. Gene-specific differential DNA methylation and chronic arsenic exposure in an epigenome-wide association study of adults in Bangladesh. Environmental Health Perspectives 123, 64, doi: 10.1289/ehp.1307884 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bozack AK et al. DNA methylation in cord blood as mediator of the association between prenatal arsenic exposure and gestational age. Epigenetics 13, 923–940, doi: 10.1080/15592294.2018.1516453 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Demanelis K. et al. Association of Arsenic Exposure with Whole Blood DNA Methylation: An Epigenome-Wide Study of Bangladeshi Adults. Environmental Health Perspectives 127, 57011–57011, doi: 10.1289/EHP3849 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bozack Anne K. et al. Locus-Specific Differential DNA Methylation and Urinary Arsenic: An Epigenome-Wide Association Study in Blood among Adults with Low-to-Moderate Arsenic Exposure. Environmental Health Perspectives 128, 067015, doi: 10.1289/EHP6263 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Niedzwiecki MM et al. Sex-specific associations of arsenic exposure with global DNA methylation and hydroxymethylation in leukocytes: Results from two studies in Bangladesh. Cancer Epidemiology, Biomarkers & Prevention 24, 1748–1757, doi: 10.1158/1055-9965.EPI-15-0432 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Howe CG et al. Associations between blood and urine arsenic concentrations and global levels of post-translational histone modifications in Bangladeshi men and women. Environmental Health Perspectives 124, 1234–1240, doi: 10.1289/ehp.1510412 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pilsner JR et al. Folate deficiency, hyperhomocysteinemia, low urinary creatinine, and hypomethylation of leukocyte DNA are risk factors for arsenic-induced skin lesions. Environmental Health Perspectives 117, 254–260 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Broberg K. et al. Arsenic exposure in early pregnancy alters genome-wide DNA methylation in cord blood, particularly in boys. Journal of Developmental Origins of Health and Disease 5, 288–298, doi: 10.1017/s2040174414000221 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kile ML et al. Effect of prenatal arsenic exposure on DNA methylation and leukocyte subpopulations in cord blood. Epigenetics 9, 774–782, doi: 10.4161/epi.28153 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Petrossian TC & Clarke SG Uncovering the human methyltransferasome. Mol Cell Proteomics 10, M110.000976, doi: 10.1074/mcp.M110.000976 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Combs GF, Gerald FC & Combs GF Jr The Vitamins. (Academic Press, 2012). [Google Scholar]
- 135.Ducker GS & Rabinowitz JD One-Carbon Metabolism in health and disease. Cell Metabolism 25, 27–42, doi: 10.1016/j.cmet.2016.08.009 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gamble MV et al. Folate and cobalamin deficiencies and hyperhomocysteinemia in Bangladesh. The American Journal of Clinical Nutrition 81, 1372–1377 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brosnan JT, da Silva RP & Brosnan ME The metabolic burden of creatine synthesis. Amino Acids 40, 1325–1331, doi: 10.1007/s00726-011-0853-y (2011). [DOI] [PubMed] [Google Scholar]
- 138.Mudd SH & Poole JR Labile methyl balances for normal humans on various dietary regimens. Metabolism: Clinical and Experimental 24, 721–735 (1975). [DOI] [PubMed] [Google Scholar]
- 139.Stead LM, Brosnan JT, Brosnan ME, Vance DE & Jacobs RL Is it time to reevaluate methyl balance in humans? The American Journal of Clinical Nutrition 83, 5–10 (2006). [DOI] [PubMed] [Google Scholar]
- 140.Deminice R, Portari GV, Vannucchi H. & Jordao AA Effects of creatine supplementation on homocysteine levels and lipid peroxidation in rats. The British Journal of Nutrition 102, 110–116, doi: 10.1017/s0007114508162985 (2009). [DOI] [PubMed] [Google Scholar]
- 141.Stead LM, Au KP, Jacobs RL, Brosnan ME & Brosnan JT Methylation demand and homocysteine metabolism: Effects of dietary provision of creatine and guanidinoacetate. American Journal of Physiology: Endocrinology and Metabolism 281, E1095–1100 (2001). [DOI] [PubMed] [Google Scholar]
- 142.Taes YEC et al. Creatine supplementation decreases homocysteine in an animal model of uremia. Kidney International 64, 1331–1337, doi: 10.1046/j.1523-1755.2003.00206.x (2003). [DOI] [PubMed] [Google Scholar]
- 143.Brosnan JT, Jacobs RL, Stead LM & Brosnan ME Methylation demand: a key determinant of homocysteine metabolism. Acta Biochim Pol 51, 405–413 (2004). [PubMed] [Google Scholar]
- 144.Schalinske KL & Steele RD Quantitation of carbon flow through the hepatic folate-dependent one-carbon pool in rats. Arch Biochem Biophys 271, 49–55, doi: 10.1016/0003-9861(89)90254-3 (1989). [DOI] [PubMed] [Google Scholar]
- 145.Gamble MV & Hall MN Relationship of Creatinine and Nutrition with Arsenic Metabolism. Environmental Health Perspectives 120, a145–a146, doi:doi: 10.1289/ehp.1104807 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vahter M. & Marafante E. Effects of low dietary intake of methionine, choline or proteins on the biotransformation of arsenite in the rabbit. Toxicology Letters 37, 41–46 (1987). [DOI] [PubMed] [Google Scholar]
- 147.Tice RR, Yager JW, Andrews P. & Crecelius E. Effect of hepatic methyl donor status on urinary excretion and DNA damage in B6C3F1 mice treated with sodium arsenite. Mutation Research 386, 315–334 (1997). [DOI] [PubMed] [Google Scholar]
- 148.Spiegelstein O. et al. Developmental consequences of in utero sodium arsenate exposure in mice with folate transport deficiencies. Toxicology and Applied Pharmacology 203, 18–26, doi: 10.1016/j.taap.2004.07.006 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Spiegelstein O. et al. Effects of dietary folate intake and folate binding protein-1 (Folbp1) on urinary speciation of sodium arsenate in mice. Toxicology Letters 145, 167–174, doi: 10.1016/S0378-4274(03)00307-2 (2003). [DOI] [PubMed] [Google Scholar]
- 150.Spiegelstein O. et al. Effects of dietary folate intake and folate binding protein-2 (Folbp2) on urinary speciation of sodium arsenate in mice. Environmental Toxicology and Pharmacology 19, 1–7, doi: 10.1016/j.etap.2004.01.007 (2005). [DOI] [PubMed] [Google Scholar]
- 151.Wlodarczyk B. et al. Arsenic-induced congenital malformations in genetically susceptible folate binding protein-2 knockout mice. Toxicology and Applied Pharmacology 177, 238–246, doi: 10.1006/taap.2001.9303 (2001). [DOI] [PubMed] [Google Scholar]
- 152.Mazumdar M. et al. Arsenic is associated with reduced effect of folic acid in myelomeningocele prevention: A case control study in Bangladesh. Environmental Health 14, 34, doi: 10.1186/s12940-015-0020-0 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tauheed J. et al. Associations between post translational histone modifications, myelomeningocele risk, environmental arsenic exposure, and folate deficiency among participants in a case control study in Bangladesh. Epigenetics 12, 484–491, doi: 10.1080/15592294.2017.1312238 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Koller BH et al. Arsenic Metabolism in Mice Carrying a BORCS7/AS3MT Locus Humanized by Syntenic Replacement. Environmental Health Perspectives 128, 87003, doi: 10.1289/ehp6943 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.van Tongeren JH, Kunst A, Majoor CL & Schillings PH Folicacid deficiency in chronic arsenic poisoning. Lancet 1, 784–786, doi: 10.1016/s0140-6736(65)92956-9 (1965). [DOI] [PubMed] [Google Scholar]
- 156.Brouwer OF et al. Increased neurotoxicity of arsenic in methylenetetrahydrofolate reductase deficiency. Clinical Neurology and Neurosurgery 94, 307–310, doi: 10.1016/0303-8467(92)90179-7 (1992). [DOI] [PubMed] [Google Scholar]
- 157.Chung JS et al. Family correlations of arsenic methylation patterns in children and parents exposed to high concentrations of arsenic in drinking water. Environmental Health Perspectives 110, 729–733 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Basu A. et al. Creatinine, diet, micronutrients, and arsenic methylation in West Bengal, India. Environmental Health Perspectives 119, 1308–1313 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Steinmaus C. et al. Dietary intake and arsenic methylation in a U.S. population. Environmental Health Perspectives 113, 1153–1159 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gamble MV et al. Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in Bangladesh. Environmental Health Perspectives 113, 1683–1688 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gamble MV et al. Folate and arsenic metabolism: A double-blind, placebo-controlled folic acid-supplementation trial in Bangladesh. The American Journal of Clinical Nutrition 84, 1093–1101 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Peters BA et al. Folic acid and creatine as therapeutic approaches to lower blood arsenic: A randomized controlled trial. Environmental Health Perspectives 123, 1294 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bozack A. et al. Folic acid supplementation enhances arsenic methylation: Results from a folic acid and creatine supplementation trial in Bangladesh. American Journal of Clinical Nutrition In Review (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bozack AHM, Liu X; Ilievski V; Lomax-Luu A; Parvez F; Shahriar H; Islam T; Graziano J; Gamble M. Folic acid effects blood arsenic metabolites: Results from the Folic Acid and Creatine Trial in Bangladesh. Environmental Epidemiology 3, 39, doi: 10.1097/01.Ee9.0000606056.51832.73 (2019). [DOI] [Google Scholar]
- 165.López-Carrillo L. et al. Dietary micronutrient intake and its relationship with arsenic metabolism in Mexican women. Environmental Research 151, 445–450, doi: 10.1016/j.envres.2016.08.015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kurzius-Spencer M. et al. Nutrients in one-carbon metabolism and urinary arsenic methylation in the National Health and Nutrition Examination Survey (NHANES) 2003–2004. The Science of the Total Environment 607–608, 381–390, doi: 10.1016/j.scitotenv.2017.07.019 (2017). [DOI] [PubMed] [Google Scholar]
- 167.Spratlen MJ et al. Arsenic metabolism and one-carbon metabolism at low-moderate arsenic exposure: Evidence from the Strong Heart Study. Food and Chemical Toxicology 105, 387–397, doi: 10.1016/j.fct.2017.05.004 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Heck JE et al. Consumption of folate-related nutrients and metabolism of arsenic in Bangladesh. American Journal of Clinical Nutrition 85, 1367–1374 (2007). [DOI] [PubMed] [Google Scholar]
- 169.Skröder Löveborn H, Kippler M, Lu Y. & Ahmed S. Arsenic Metabolism in Children Differs From That in Adults. Toxicological sciences 152, 29–39, doi: 10.1093/toxsci/kfw060 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hall MN et al. Folate, cobalamin, cysteine, homocysteine, and arsenic metabolism among children in Bangladesh. Environmental Health Perspectives 117, 825–831, doi: 10.1289/ehp.0800164 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chowdhury UK et al. Pattern of excretion of arsenic compounds [arsenite, arsenate, MMA(V), DMA(V)] in urine of children compared to adults from an arsenic exposed area in Bangladesh. J Environ Sci Health A Tox Hazard Subst Environ Eng 38, 87–113, doi: 10.1081/ese-120016883 (2003). [DOI] [PubMed] [Google Scholar]
- 172.Sun G. et al. Urinary arsenic metabolites in children and adults exposed to arsenic in drinking water in Inner Mongolia, China. Environmental Health Perspectives 115, 648–652, doi: 10.1289/ehp.9271 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Saxena R. et al. Nutrition and Arsenic Methylation in Bangladeshi Adolescents. (Environmental Research., In Press.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sadre-Marandi F, Dahdoul T, Reed MC & Nijhout HF Sex differences in hepatic one-carbon metabolism. BMC Systems Biology 12, 89, doi: 10.1186/s12918-018-0621-7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zeisel SH Importance of methyl donors during reproduction. The American Journal of Clinical Nutrition 89, 673S–677S, doi: 10.3945/ajcn.2008.26811D (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li L. et al. Nutritional status has marginal influence on the metabolism of inorganic arsenic in pregnant Bangladeshi women. Environmental Health Perspectives 116, 315–321 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Laine JE et al. Maternal one carbon metabolism and arsenic methylation in a pregnancy cohort in Mexico. J Expo Sci Environ Epidemiol 28, 505–514, doi: 10.1038/s41370-018-0041-1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gardner RM et al. Arsenic methylation efficiency increases during the first trimester of pregnancy independent of folate status. Reproductive Toxicology 31, 210–218, doi: 10.1016/j.reprotox.2010.11.002 (2011). [DOI] [PubMed] [Google Scholar]
- 179.Kile ML et al. Variability in biomarkers of arsenic exposure and metabolism in adults over time. Environmental Health Perspectives 117, 455–460, doi: 10.1289/ehp.11251 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Peters BA et al. Low-dose creatine supplementation lowers plasma guanidinoacetate, but not plasma homocysteine, in a double-blind, randomized, placebo-controlled trial. The Journal of Nutrition 145, 2245–2252, doi: 10.3945/jn.115.216739 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bozack AK et al. Folic acid supplementation enhances arsenic methylation: results from a folic acid and creatine supplementation randomized controlled trial in Bangladesh. The American Journal of Clinical Nutrition 109, 380–391, doi: 10.1093/ajcn/nqy148 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bozack A. et al. Betaine and choline status modify the effects of folic acid and creatine supplementation on arsenic methylation in a randomized controlled trial of Bangladeshi adults. European journal of nutrition, doi: 10.1007/s00394-020-02377-z (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kennedy EP Metabolism of Lipides. Annual Review of Biochemistry 26, 119–148, doi: 10.1146/annurev.bi.26.070157.001003 (1957). [DOI] [PubMed] [Google Scholar]
- 184.Cui Z. & Vance DE Expression of phosphatidylethanolamine N-methyltransferase-2 is markedly enhanced in long term choline-deficient rats. The Journal of Biological Chemistry 271, 2839–2843, doi: 10.1074/jbc.271.5.2839 (1996). [DOI] [PubMed] [Google Scholar]
- 185.Huang MC, Douillet C, Dover EN & Stýblo M. Prenatal arsenic exposure and dietary folate and methylcobalamin supplementation alter the metabolic phenotype of C57BL/6J mice in a sex-specific manner. Archives of Toxicology 92, 1925–1937, doi: 10.1007/s00204-018-2206-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hall MN et al. Influence of cobalamin on arsenic metabolism in Bangladesh. Environmental Health Perspectives 117, 1724–1729 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Vance JE & Vance DE Phospholipid biosynthesis in mammalian cells. Biochemistry and Cell Biology 82, 113–128, doi: 10.1139/o03-073 (2004). [DOI] [PubMed] [Google Scholar]
- 188.Zeisel SH & Warrier M. Trimethylamine N-Oxide, the microbiome, and heart and kidney disease. Annual Review of Nutrition 37, 157–181, doi: 10.1146/annurev-nutr-071816-064732 (2017). [DOI] [PubMed] [Google Scholar]
- 189.Tang WHW et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. The New England Journal of Medicine 368, 1575–1584, doi: 10.1056/NEJMoa1109400 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sauve AA NAD+ and vitamin B3: from metabolism to therapies. J Pharmacol Exp Ther 324, 883–893, doi: 10.1124/jpet.107.120758 (2008). [DOI] [PubMed] [Google Scholar]
- 191.Pierce BL et al. A missense variant in FTCD is associated with arsenic metabolism and toxicity phenotypes in Bangladesh. PLoS Genet 15, e1007984-e1007984, doi: 10.1371/journal.pgen.1007984 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Engström KS et al. Arsenic metabolism is influenced by polymorphisms in genes involved in one-carbon metabolism and reduction reactions. Mutation Research 667, 4–14, doi: 10.1016/j.mrfmmm.2008.07.003 (2009). [DOI] [PubMed] [Google Scholar]
- 193.Porter KE et al. Association of genetic variation in cystathionine-β-synthase and arsenic metabolism. Environmental Research 110, 580–587, doi: 10.1016/j.envres.2010.05.001 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gamboa-Loira B. et al. Arsenic methylation capacity in relation to nutrient intake and genetic polymorphisms in one-carbon metabolism. Environmental Research 164, 18–23, doi: 10.1016/j.envres.2018.01.050 (2018). [DOI] [PubMed] [Google Scholar]
- 195.González-Martínez F. et al. As3MT and GST Polymorphisms Influencing Arsenic Metabolism in Human Exposure to Drinking Groundwater. International Journal of Molecular Sciences 21, doi: 10.3390/ijms21144832 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mitra SR et al. Nutritional factors and susceptibility to arsenic-caused skin lesions in West Bengal, India. Environmental Health Perspectives 112, 1104–1109, doi: 10.1289/EHP.6841 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Carreras CW & Santi DV The catalytic mechanism and structure of thymidylate synthase. Annual Review of Biochemistry 64, 721–762, doi: 10.1146/annurev.bi.64.070195.003445 (1995). [DOI] [PubMed] [Google Scholar]
- 198.Koutros S. et al. Potential effect modifiers of the arsenic-bladder cancer risk relationship. Int J Cancer 143, 2640–2646, doi: 10.1002/ijc.31720 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Howe CG et al. Sex-Specific Associations between One-Carbon Metabolism Indices and Posttranslational Histone Modifications in Arsenic-Exposed Bangladeshi Adults. Cancer Epidemiology, Biomarkers, & Prevention 26, 261–269, doi: 10.1158/1055-9965.Epi-16-0202 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Navas-Acien A, Silbergeld E, Pastor-Barriuso R. & Guallar E. Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA 300, doi: 10.1001/jama.300.7.814 (2008). [DOI] [PubMed] [Google Scholar]
- 201.Spratlen MJ et al. Targeted metabolomics to understand the association between arsenic metabolism and diabetes-related outcomes: Preliminary evidence from the Strong Heart Family Study. Environmental research 168, 146–157, doi: 10.1016/j.envres.2018.09.034 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Spratlen MJ et al. Arsenic, one carbon metabolism and diabetes-related outcomes in the Strong Heart Family Study. Environment international 121, 728–740, doi: 10.1016/j.envint.2018.09.048 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Dabelea D. et al. Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH Case-Control Study. Diabetes Care 31, 1422–1426, doi: 10.2337/dc07-2417 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kelstrup L. et al. Insulin resistance and impaired pancreatic β-cell function in adult offspring of women with diabetes in pregnancy. J Clin Endocrinol Metab 98, 3793–3801, doi: 10.1210/jc.2013-1536 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Nehring I, Chmitorz A, Reulen H, von Kries R. & Ensenauer R. Gestational diabetes predicts the risk of childhood overweight and abdominal circumference independent of maternal obesity. Diabet Med 30, 1449–1456, doi: 10.1111/dme.12286 (2013). [DOI] [PubMed] [Google Scholar]
- 206.Silverman BL, Metzger BE, Cho NH & Loeb CA Impaired glucose tolerance in adolescent offspring of diabetic mothers. Relationship to fetal hyperinsulinism. Diabetes Care 18, 611–617, doi: 10.2337/diacare.18.5.611 (1995). [DOI] [PubMed] [Google Scholar]
- 207.Navas-Acien A. et al. Early-Life Arsenic Exposure, Nutritional Status, and Adult Diabetes Risk. Current Diabetes Reports 19, 147, doi: 10.1007/s11892-019-1272-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lawley SD et al. Mathematical model insights into arsenic detoxification. Theoretical Biology & Medical Modelling 8, 31, doi: 10.1186/1742-4682-8-31 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Reed MC, Gamble MV, Hall MN & Nijhout HF Mathematical analysis of the regulation of competing methyltransferases. BMC Systems Biology 9, 69, doi: 10.1186/s12918-015-0215-6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]