FIGURE 3.
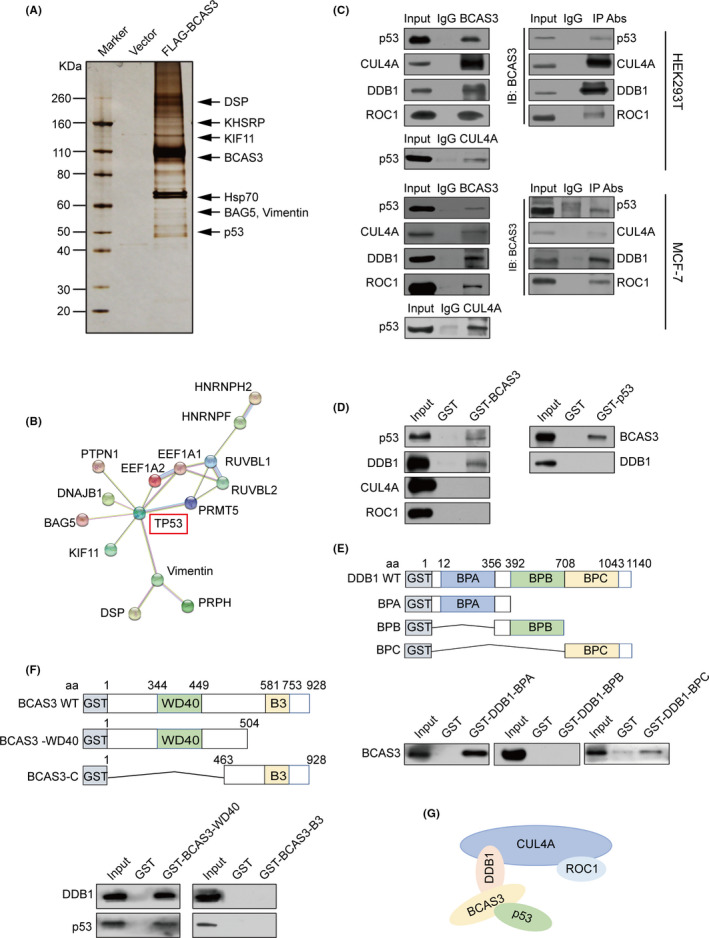
BCAS3 is physically associated with CRL4A complex and p53. (A). BCAS3 physically interacts with p53 in vivo. Cellular lysates are immunoprecipitated with anti‐FLAG. FLAG peptide is applied to elute the FLAG‐tagged proteins. Proteins are fractioned, stained and sequenced by LC‐MS/MS. (B). PPI analysis of BCAS3‐associated proteins. (C). Association of BCAS3 with p53 and CRL4A complex in HEK 293T and MCF‐7 cells. Whole‐cell lysates are immunoprecipitated with specific antibodies and then immunoblotted with indicated antibodies. (D). Co‐incubate the GST fusion protein and the transcribed‐translated protein in vitro for GST pull‐down. (E). BCAS3 directly binds to the BPA domain of DDB1 in vitro. Co‐incubate the GST fusion constructs of DDB1 and the transcribed‐translated BCAS3 protein in vitro for GST pull‐down. (F). WD40 domain of BCAS3 is responsible for interaction with DDB1 and p53. GST pull‐down assays are conducted using constructs of BCAS3 and in vitro‐translated DDB1 and p53 proteins. (G). Schematic diagram of molecular basis between BCAS3, p53 and CRL4A complex
