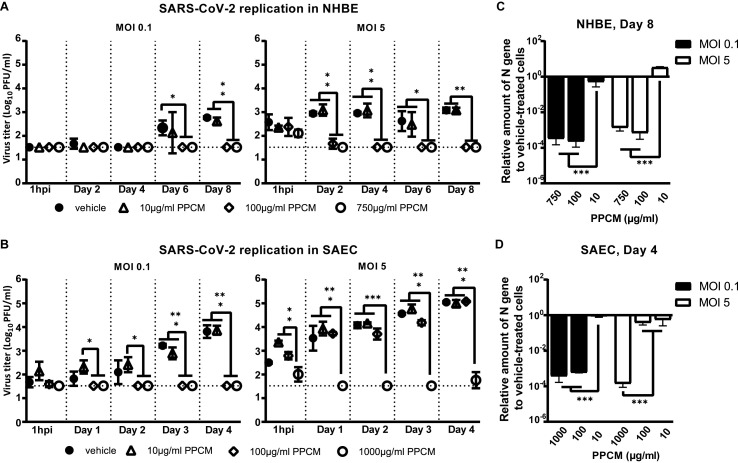
Fig. 5.
PPCM antiviral effect against SARS-CoV-2 in NHBE and SAEC. Virus replication kinetics in (A) NHBE or (B) SAEC following a (left) low and (right) high infective dose inoculation. Assays performed with PPCM treatments (0–1000 μg/ml) starting 1h prior infection and maintained daily for up to 8 days, as described in materials and methods. Detection and relative quantification of SARS-CoV-2 nucleocapsid (N) gene expression in PPCM-versus vehicle-treated (C) NHBE or (D) SAEC cells at day 8 and 4 pi, respectively. (A–D) Results are expressed as the average of biological duplicates or triplicates, and error bars represent standard errors. (A, B) The horizontal dotted lines correspond to the detection limit. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (ANOVA, Tukey's test).