Abstract
Background:
Severe food allergic reactions can be life-threatening or fatal, and are experienced by up to 40% of children with food allergies, with adolescents at greatest risk. There are no comprehensive measures to assess food allergy management behaviors that could prevent allergic reactions.
Objective:
The objectives of this paper are to describe food allergy self-management behaviors as reported by adolescents on a 24-hour recall measure and identify related factors.
Methods:
Adolescents ages 10-14 years with IgE-mediated food allergy completed the Food Allergy Management 24-Hour Recall (FAM-24) as an interview. Participants answered questions about each food they ate the previous day and food allergy self-management behaviors.
Results:
Participants were a diverse sample (28% Caucasian) of 101 adolescents (M age = 11.80 years; 53% male). Most meals/snacks (76%) were observed by adults. Epinephrine auto-injectors (EAI) were reportedly available for almost all meals/snacks (93%). Almost all foods had been eaten before (95%) and were verified as allergen free (92%). Thirty-five percent of the time past experience with the food was the only method used to verify safety. Child age, number of food allergies, or time since allergic reaction were not related to self-management behavior. EAI availability and ingredient verification were most common at home and in school; adult observation was least likely in the home.
Conclusion:
Adolescents reported that EAIs were frequently available, but relied on past experience with food to determine safety. Appropriate assessment of food safety should be a primary intervention target. The FAM-24 may be a useful tool to assess and track FA self-management.
Keywords: food allergy, adolescents, adherence, assessment, 24-hour recall
Introduction
The prevalence of food allergies (FA) has been on a rapid rise over the last few decades, with increase in childhood and adolescent cases. Research shows that 2.3-8% of individuals 0-19 years of age have a FA proven by oral food challenge1,2 or as detected by food-specific IgE.3 Furthermore, parent-proxy reports estimate FA prevalence at 7.6%, of which 42.3% children have at least one severe FA and 39.9% have multiple FA.4 Severe FA reactions can be life-threatening or even fatal.5 Up to 42% of children with FA have at least one lifetime FA-related emergency department visit with 19% reporting at least one visit in the previous year.4 Adolescents, in particular, are at higher risk of FA fatalities due to food-induced anaphylaxis.6 The primary reason for food-related allergic reactions among adolescents is unintentional exposure, mostly by cross-contact, which is when an allergen comes into contact with a safe food.7 However, about 10% of exposures are to known allergens, with the most common reasons being the child had not experienced a serious allergic reaction in the past, they were unaware of the risk, they knew the risk and decided to take the risk, or they did not believe the seriousness of food allergy.7
Although there is now an FDA-approved oral immunotherapy treatment for peanut allergy, the primary method of allergic reaction prevention for most patients with food allergy is total allergen avoidance. This regimen involves several complex behaviors and lifestyle changes including understanding allergic triggers, investigating the presence of allergens in food items, avoidance of such items, avoidance of situations where accidental ingestion may be possible, and monitoring food cross-contact. In addition, FA management requires knowledge about when and how to treat an allergic reaction and carrying medications (e.g. epinephrine auto-injectors (EAI)) in order to be prepared for any allergic reactions that occur.
Adherence to health-care behaviors in chronic illness is often difficult and complicated by factors that promote it or act as barriers. The Health Belief Model was developed to understand these factors and proposes that adherence to health-care behaviors is associated with demographic variables, psychosocial factors, knowledge about the disease, perceived threat of disease, and cues and reminders of disease factors.8 The risk of FA-related fatalities may be higher in adolescence for a variety of reasons that are understood in the context of the Health Belief Model. Risk for an allergic reaction is greater because adolescents eat more often outside of the home and may engage in risk-taking behavior (e.g., eating food with precautionary allergen labels).9–11 In teenage years, parental oversight and supervision decreases, which results in teens needing to make their own decisions about what they consume. Adolescents are expected to identify foods they need to avoid and recognize potential symptoms and course of action needed for exposures,10 yet many adolescents cannot identify anaphylaxis or when to use epinephrine.1,12,13 Adolescents may perceive carrying EAIs to be burdensome, which can significantly impact their compliance.14 Difficulties with adherence may worsen in college years as adolescents gain more freedom and have more control over their health behaviors.15 If not prepared adequately, adolescents may struggle with managing these needs and inadvertently increase their risk of allergen exposure.
The healthcare behaviors necessary to manage FAs can be categorized as dietary allergen avoidance and access to pharmacological intervention to treat food-induced allergic reactions.16 However, measurement of adherence to allergen avoidance and EAI availability remains challenging. In other diseases requiring dietary adherence, such as Chronic Kidney Disease and type 1 diabetes, dietary adherence can be measured with laboratory tests for phosphorus and potassium, and blood glucose levels and HbA1c, respectively. Yet laboratory testing and biomarkers are not available for FA, complicating quantitative measurement of adherence. The majority of research regarding FA management adherence uses single questions or a few items, such as whether patients read labels, avoid allergens, avoid “may contain” items, and/or ask about ingredients;10,17 whether or not they carry EAIs;17 or how often they carry an EAI.18 Results from these studies provide varying estimates of how frequently adolescents avoid their allergens (85% “tried” in one report,12 20-37% eat foods with precautionary allergy labels in another,19 and 20% knowingly ate foods with allergens in another10) and how many adolescents always carry their EAIs (41-80%10,12,18). However, these reports do not provide a full picture of daily FA self-management and a more comprehensive assessment tool is needed.
Adherence in diet-related disease management may be best measured from patient and family’s reports such as food diaries, 14-day food records, 24-hour recall, and diet history, which all have various degrees of validity. Although underreporting is prevalent in all methods of dietary assessment,20 24-hour recalls and diet history interviews are the most accurate among children21, and have been used in pediatric overweight/obese,21 hypertension,20 and diabetes samples.22 Furthermore, web-based and interviewer administered 24-hour dietary recalls have been shown to be valid indicators of food intake based on objective biomarkers such as protein, potassium, total sugar, and total energy intake.23 Although 24-hour recall has been used in FA research to study diet patterns,24 it has not been used, to our knowledge, to assess FA self-management behaviors. The aims of this paper are to describe FA self-management behaviors as reported by adolescents on a 24-hour recall measure and identify factors related to them.
Methods
Development of the Food Allergy Management 24-Hour Recall (FAM-24)
The FAM-24 was designed by the study team to document FA management behaviors during the previous day. It is based on 24-hour recall measures that have been utilized with populations diagnosed with other chronic illness, such as type 1 diabetes,25–27 which have demonstrated success as indicators of adherence to disease management guidelines. The FAM-24 was adapted from a type 1 diabetes 24-hour recall, developed by Johnson and colleagues in 1986, that assesses adolescents’ daily blood glucose checks and insulin injections, including when they were conducted, where, and by whom. For this study, an expert review group composed of an allergist, an allergy nurse, and a child health psychologist with FA expertise developed a list of daily FA self-management behaviors based on guidelines for FA management.16 Two categories of FA self-management were identified: EAI availability and allergen avoidance. Contextual factors were also identified that would further elucidate adolescents’ FA self-management experiences. Within the EAI availability category, whether or not an EAI was available and its location were identified as most important. Within the allergen avoidance category, past experience with the food, how the food was verified as allergen-free, who verified it, and the presence of precautionary allergy labeling were identified as most important. Meal location, adult observation, and experience of an allergic reaction were also identified as important contextual factors. The team then translated the categories into an interview format. Additional allergists and nurses within the allergy clinical team and a child health psychologist who used the type 1 diabetes 24-hour recall interview for research purposes reviewed the FAM-24 draft and provided feedback. Adolescents with FA, recruited from the FA clinic, then completed the FAM-24 draft and provided feedback. The FAM-24 was updated based on their feedback. For example, adolescents were asked if the food they ate had precautionary allergy labeling, and if so, for specific language (e.g., may contain “xx”, manufactured in the same facility as “xx”). However, very few adolescents could recall if the food had such labeling and even fewer knew the specific language. Thus, these items were not included in the FAM-24 due to concern about inaccurate reporting.
In the final version of the FAM-24, adolescents answered a series of questions about each food they ate during the previous day. Participants reported on the time of the meal/snack, where they ate (e.g., school, home, a restaurant), whether an adult was present, whether EAI was available, and if so, where it was located (e.g., on person, in another room). They also reported if they had an allergic reaction. For each individual food eaten during the meal/snack, participants reported what the food was, whether they ate it before, whether it was verified as allergen-free, how it was verified (e.g., prepared at home, read the label, asked about ingredients), and who verified it (e.g., child, parent, teacher, another adult). Participants could endorse multiple methods of allergen-free verification and multiple people who completed the verification.
Procedure
Participants.
This project is part of a larger longitudinal study of adolescents with FAs and their primary caregiver. This study was IRB approved by the home institution. Participants were English-fluent patients age 10-14 years at a pediatric FA clinic being treated for a diagnosis of at least one of the 8 most common IgE-mediated FAs (peanut, tree nut, cow’s milk, egg, wheat, soy, fish, and shellfish) and had an EAI prescription. Participants were excluded if they had a diagnosis of celiac disease, a non-atopic chronic illness, or developmental disabilities or cognitive limitations. However, eligibility criteria included children with allergic diseases highly comorbid with FA (asthma, allergic rhinitis, atopic dermatitis, drug allergies, and eosinophilic esophagitis).
Participant Recruitment.
Participants were consecutively recruited from FA clinics until the baseline recruitment goal for the larger longitudinal study of 100 participants was met. The research team identified potential participants through referral by a clinic allergist or identification via prospective medical chart reviews of patients with an upcoming clinic appointment. A member of the study team contacted the patient’s caregiver by phone or met with the family in-person at an allergy appointment to discuss study requirements and conduct an initial screening. Eligible and interested primary caregivers’ written consent was obtained. Written assent was obtained for participants between 12 and 14 years, and verbal assent was obtained for all child participants.
Questionnaire Completion.
Participants completed the FAM-24 with a member of the study team in interview format, either in-person after their enrollment or over the phone within two months of completing their other study questionnaires. The participant was informed that they should answer the questionnaire on their own, but could have limited assistance from their primary caregiver. The study team member who conducted the interview recorded responses on a paper form and field notes on additional elaborating information provided by the respondent. All responses were then entered into REDCap, a secure web application for building and managing online surveys and databases.28
Sociodemographic and Medical Information.
Demographic information was obtained through the surveys completed by the participants and their primary caregiver for the larger study, either via an online or paper survey. These data included child gender, age, race, and ethnicity, and annual household income. Participants also completed a Medical Information questionnaire, which identified their specific FAs, the date of FA diagnoses, and other medical diagnoses. Diagnoses were confirmed by a medical chart review conducted by the study team.
Statistical Analyses
All analyses were conducted in SPSS Version 26.0. To address the first aim, description of FA management adherence as reported by adolescents, two databases were created. The first database was formatted to permit individual participant-level analyses, with each participant as a different case. From this database, descriptive statistics were conducted to assess overarching demographic and medical variables. Individual-level variables were also calculated, such as percent of the time that meals were observed by an adult for each participant, that EAI was available, and ingredients were verified as allergen-free. Frequencies and means were calculated for each of those variables. The second database was formatted to permit meal-level and food-level analyses, with each food entry as a different case. From this second database, overarching frequencies and means regarding meals and foods were calculated regarding meal observation, EAI availability, and ingredient verification. To address the second aim, identification of factors related to self-reported FA management behaviors, we used the first dataset to examine correlations among child age, number of FAs, and time since allergic reaction with percent of time an adult observed the meal, the percent of time EAI was available, and the percent of time the food was verified as allergen-free. Using the second database, we conducted chi-square analyses to determine the relationship of 4 categories of meal/snack location (home, school, restaurants, other places) with adult observation, EAI availability, and ingredient verification.
Results
Demographic and Medical Characteristics
Data were collected from 106 participants. Prior to conducting analyses, all FAM-24s were examined. Two participants were excluded from analyses because they indicated it was an “atypical” day, 2 participants were excluded because they did not have an EAI prescription, and 1 participant was excluded because they were diagnosed with oral allergy syndrome, not an IgE-mediated FA. Thus, data from 101 participants were included in analyses. Adolescents had an average age of 11.80 years (SD = 1.37) and were 53% male and primarily non-White (28% White) and non-Hispanic (84%). The most common FAs were tree nuts (79%) and peanut (72%), although all of the top 8 FAs were represented. Participants were diagnosed with an average 2.98 FAs (SD = 2.21; Range = 1-12). Mean age of FA diagnosis was 3.18 years (SD = 2.98). See Table 1 for additional demographic information and Table 2 for additional medical information. Almost half (43%) of participants reported that their most recent allergic reaction was in the past year; 2% reported they had never experienced an allergic reaction and 13% were not sure if/when the most recent reaction occurred. Two-thirds of the sample (64%) had experienced an allergic reaction at home; one quarter (23%) had experienced one at school/day care. Regarding EAI use, 34% reported that someone administered an EAI and 7% reported self-administration. Nearly half of participants (46%) remembered going to an emergency room; 6% reported staying overnight.
Table 1.
Participant Demographic Information (n=101)
| Percentage | M | SD | Range | |
|---|---|---|---|---|
| Child/Family Demographics | ||||
| Child age (months) | 11.83 | 1.37 | 10.00 - 15.00 | |
| Child sex (% male) | 52.4 | |||
| Child race | ||||
| Black | 37.9 | |||
| White | 28.2 | |||
| Asian | 5.8 | |||
| American Indian or Alaskan Native | 4.0 | |||
| Native Hawaiian or Pacific Islander | 1.0 | |||
| More than 1 race | 11.0 | |||
| Other | 8.0 | |||
| Child ethnicity (% non-Hispanic) | 84.0 | |||
| Household annual income | ||||
| < $50,000 | 21.0 | |||
| $50,000-110,000 | 17.0 | |||
| $110,000-200,000 | 23.0 | |||
| > $200,000 | 23.0 | |||
Table 2.
Participant Medical Information (n=101)
| Percentage | M | SD | Range | |
|---|---|---|---|---|
| Total Number of Food Allergies | 2.98 | 2.21 | 1-12 | |
| Specific Food Allergens | ||||
| Peanut | 69.9 | |||
| Tree Nut | 74.8 | |||
| Milk (Baked/Direct) | 7.8/10.7 | |||
| Egg (Baked/Direct) | 10.7/18.4 | |||
| Wheat | 5.8 | |||
| Soy | 8.7 | |||
| Fish | 22.3 | |||
| Shellfish | 28.2 | |||
| Sesame | 18.4 | |||
| Other | 19.6 | |||
| Food Allergy Experiences | ||||
| Child has epinephrine prescription | 100 | |||
| Child self-administered epinephrine | 6.8 | |||
| Epinephrine administered by someone else | 33.0 | |||
| Ever had allergic reaction at home | 62.1 | |||
| Ever had allergic reaction at school/day care | 20.4 | |||
| Required transport to the Emergency Room for allergic reaction | 43.7 | |||
| Required hospital admission for allergic reaction | 5.8 | |||
| Other Medical Diagnoses | ||||
| Eczema | 58.3 | |||
| Asthma | 51.5 | |||
| Environmental Allergies | 45.6 | |||
| Oral Allergy Syndrome | 8.7 | |||
| Drug Allergy | 8.7 | |||
| Eosinophilic Esophagitis | 6.8 | |||
| Lactose Intolerance | 2.9 | |||
| Eosinophilic Gastritis/Gastrointestinal Disorder | 2.1 | |||
Meal/Snack Overview
Participants reported eating 289 meals (Mean per participant = 2.81; SD = 0.46) and 89 snacks (Mean per participant = 1.34; SD = 0.59), and a total of 760 foods. Two-thirds of meals/snacks were eaten at home (66%; n = 248); other locations included school (18%; n = 69), other relatives’ homes (3%, n = 13), restaurants (2%, n = 8), friends’ homes (2%, n = 6), and other places such as in the car (9%, n = 33). One participant reported that they experienced throat itching after eating an orange, which was not one of their reported IgE-mediated FAs. The participant reported that they did not inform anyone of their symptoms and “drank a lot of water” to manage them. No other symptoms were reported after any meals or snacks. Adult observation, EAI availability, and ingredient verification were not significantly correlated, ps > .05.
Adult Observation
Adults observed 76% of meals/snacks (n = 286). Most meals/snacks were observed by a parent (68%, n = 194), another unrelated adult (15%, n = 44), another relative (14%, n = 40), or a teacher (14%, n = 40). (Total is more than 100% because more than one adult could observe the meal.) There was variability among participants regarding the percent of meals/snacks with adult observation (Mean percent = 75%, SD = 0.26; Range = 0-100%). See Figure 1. Most participants reported that meals were observed 49-100% of the time, but some participants (n = 13) reported that their meals were observed by an adult less than half the time. Child age, number of FAs, and time since allergic reaction were not significantly correlated with adult observation of the meal/snack, all ps > .05. See Table 3. However, results of a chi-square analysis indicated that the location of the meal/snack was related to adult observation, such that adult observation was less likely in the home than in school, restaurants, and other locations, X2(3, N=375) = 11.96, p =.008. See Table 4.
Figure 1.
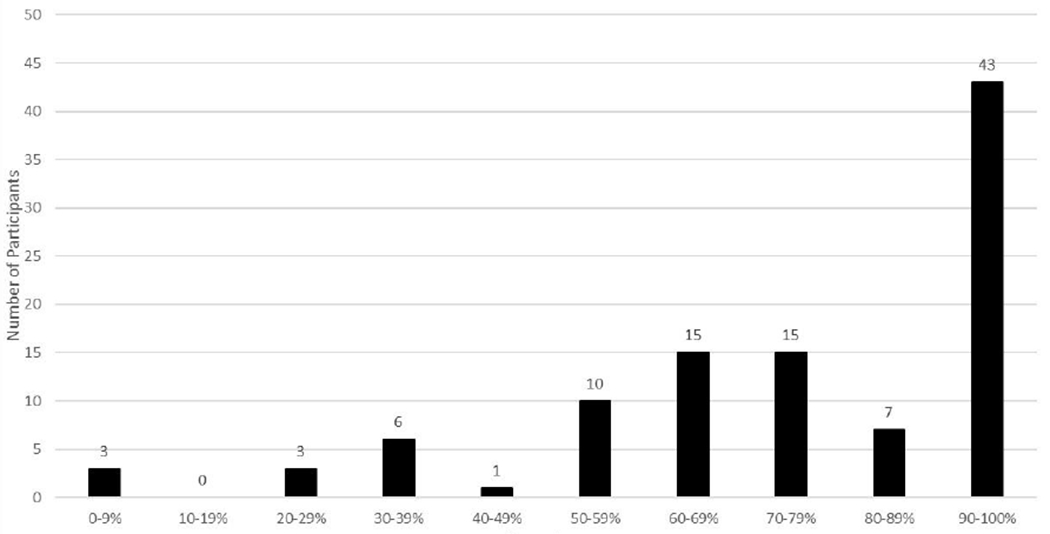
Percentage of Meals/Snacks Observed By an Adult (N=101)
Table 3.
Correlations among demographic/medical variables and adult observation, EAI availability, and ingredient verification (n = 101)
| Child Age | Number of Food Allergies | Time Since Allergic Reaction | 1 | 2 | |
|---|---|---|---|---|---|
| 1. Adult Observation | −.18 | −.02 | .14 | ||
| 2. EAI Availability | .10 | .14 | .09 | −.13 | |
| 3. Ingredient Verification | −.08 | .08 | −.05 | −.04 | .07 |
Note. EAI = epinephrine auto-injector
Table 4.
Adherence Data by Location (N=101)
| Home | School | Restaurant | Relative’s home | Friend’s home | Other places | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Percent Yes (n) |
N | Percent Yes (n) |
N | Percent Yes (n) |
N | Percent Yes (n) |
N | Percent Yes (n) |
N | Percent Yes (n) |
|
| Adult observation | 247 | 70.9 (175) | 69 | 82.6 (57) | 8 | 100.0 (8) | 13 | 84.6 (11) | 6 | 100.0 (6) | 32 | 87.5 (28) |
| EAI available | 247 | 98.0 (242) | 69 | 92.5 (62) | 8 | 75.0 (6) | 13 | 92.3 (12) | 6 | 66.7 (4) | 32 | 63.6 (21) |
| Food Item Verified as Allergen-Free: * | ||||||||||||
| Verified in a way that was not “safe in the past” | 436 | 69.7 (304) | 137 | 63.5 (87) | 15 | 73.3 (11) | 30 | 40.0 (12) | 15 | 33.3 (5) | 48 | 37.5 (18) |
| Food was prepared at home | 474 | 32.7 (155) | 164 | 15.2 (25) | 16 | 0.0 (0) | 31 | 19.4 (6) | 15 | 6.7 (1) | 57 | 10.5 (6) |
| Read the label | 474 | 32.3 (153) | 164 | 38.4 (63) | 16 | 31.3 (5) | 31 | 19.4 (6) | 15 | 26.7 (4) | 57 | 17.5 (10) |
| Asked about ingredients | 474 | 3.8 (18) | 164 | 3.0 (5) | 16 | 43.8 (7) | 31 | 0.0 (0) | 15 | 0.0 (0) | 57 | 7.0 (4) |
| Food was safe in the past | 474 | 78.3 (371) | 164 | 75.6 (124) | 16 | 56.3 (9) | 31 | 90.3 (28) | 15 | 86.7 (13) | 57 | 75.4 (43) |
Percentages for each location by verification method may add up to more than 100% because participants could respond with multiple answers.
EAI = epinephrine auto-injector
Epinephrine Availability
EAI was reported as available for 93% of meals/snacks (n = 347). There was variability in where EAI was located. EAI was most frequently located in a nearby room (71%; n = 247), followed by on the child, (22%, n = 78), on the parent (16%; n = 54), in another location, such as in the car (7%, n = 26), or with another adult (1%, n = 3). (Total is more than 100% because EAI could be available in multiple locations.) There was variability among participants regarding the frequency at which EAI was available (Mean percent = 92%, SD = 0.21; Range = 0-100%). See Figure 2. Most participants reported that EAIs were available more than two-thirds of the times they ate, but two participants reported EAI was never available. Child age, number of FAs, and time since allergic reaction were not significantly correlated with EAI availability, all ps > .05. A chi-square analysis indicated that the location of the meal/snack was related to EAI availability; EAIs were most likely to be available at home and school, X2(3, N=374) = 50.05, p < .001. See Table 4.
Figure 2.
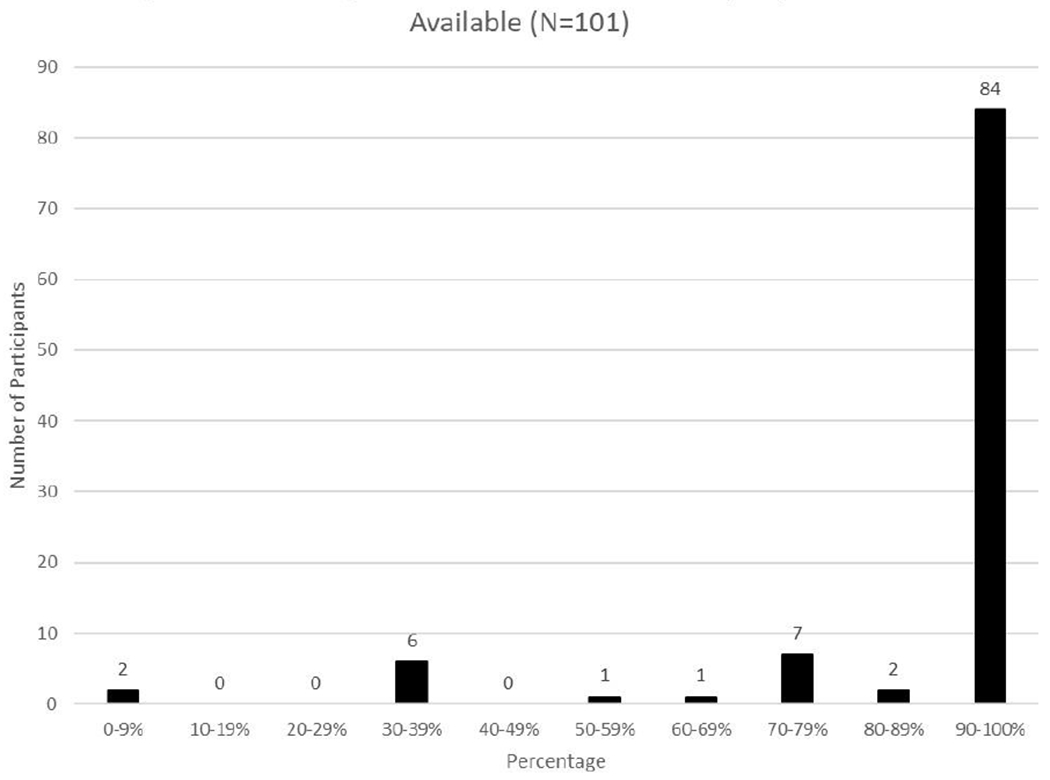
Percentage of Meals/Snacks at Which Epinephrine was Available (N=101)
Ingredient Verification
Almost all 760 foods had been eaten before (95%; n = 720) and were reportedly verified as allergen free (92%, n = 700); however, of these foods, 35% (n = 244) were only verified as allergen-free because the participant reported the food was safe in the past. Other methods of verification included that someone read the label (34%, n = 241), food was prepared at home (28%; n = 194), or someone asked about ingredients (5%; n = 34). Of the 438 foods that were verified by reading the label, preparing it at home, or asking someone about ingredients, the child most commonly verified the food was allergen-free (73%; n = 318), followed by the parent (58%, n = 252), and another relative (3%, n = 11). Teachers and other adults were responsible for ingredient verification less than 1% of the time. There was variability among participants regarding the frequency at which foods were verified as allergen-free by a method that was not presuming it was safe due to prior safety (Mean percent = 57%, SD = 0.29; Range = 0-100%). See Figure 3. This range indicates that some adolescents follow guidelines to verify foods as safe most of the time, but many use these techniques for less than half the foods they eat. Child age, number of FAs, and time since allergic reaction were not significantly correlated with verification of an ingredient by reading the label, asking about ingredients, or preparing the food at home, all ps > .05. A chi-square analysis indicated that the location of the meal/snack was related to ingredient verification, such that adolescents were most likely to report a recommended method of ingredient verification at home, school, and restaurants, X2(3, N=681) = 34.91, p < .001. See Table 4.
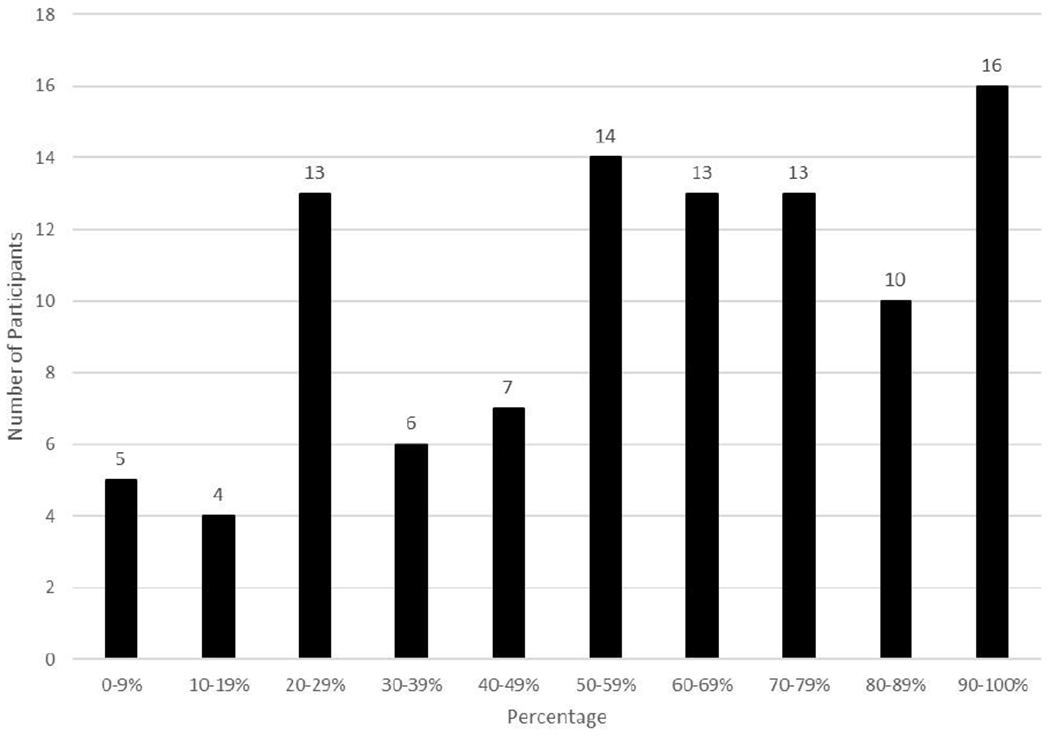
Percentage of Foods That Were Verified Allergen-Free
Discussion
The current treatment options for patients with food allergy include either total allergen avoidance or, if the patient has a peanut allergy, an FDA-approved oral immunotherapy which delivers a standardized daily dose of peanut. Treatment through total allergen avoidance can be categorized into allergen avoidance and emergency preparation behaviors, and even treatment for peanut allergy with oral immunotherapy requires both of these daily management behaviors alongside adherence to daily peanut doses. It is challenging to assess adherence to daily FA management guidelines because there are no biomarkers that approximate frequency of allergen exposure (either intentional or accidental, known or unknown) or allergic reaction. Rather, clinicians who work with patients with FA must rely on self-report, which is subject to recall bias and demand characteristics bias. This is even more complicated in pediatric allergy, when parents are the primary reporters and may be unaware of their child’s behavior, especially as their autonomy from their parents increases. Thus, it is likely that clinicians do not have an accurate understanding of their patients’ FA management or the areas in which their patients may benefit from additional assistance. Previous studies of FA management adherence among adolescents have used self-report assessments comprised of a few items regarding the frequency of carrying EAIs and reading food labels; these are a commendable place to start given the outlined challenges, but it is likely that they are only skimming the surface of the realities of daily FA management. This study represents the first evaluation of FA self-management among adolescents via a 24-hour recall measure, the FAM-24.
Participants in this study reported varying degrees of adherence to FA management guidelines, with variability most closely tied to the location of the meal/snack; other demographic and medical variables-- child age, number of FAs, and time since allergic reaction-- were not related to adult observation, EAI availability, or verification that food was allergen-free. Overall, this sample reported that EAI was available most of the times they ate, more frequently than reported in previous studies on EAI carriage.10,12,18 However, EAI availability assessment may have differed in this study from other studies and how adolescents defined “available” in this study varied; EAI were most often in a nearby room as opposed to on the child or the parent, which could lead to a delay in treatment. Epinephrine was most likely to be available at home, in school, and in the homes of relatives. Concerningly, some adolescents reported that EAIs were never available, despite the fact that this was a sample who all had EAI prescriptions, a finding comparable to other studies of adolescent adherence to EAI guidelines.10,12,18 Whether this was a true lack of EAI availability or if adolescents did not know about the EAI’s location warrants further investigation. A simple point of clinical intervention may be to ensure that allergy clinicians and family openly discuss EAI locations.
Adolescents reported that almost all foods were verified as allergen-free, but a closer inspection revealed that most of the time adolescents relied on previous experience with the food to determine its safety. Given that manufacturing practices and ingredients may change over time, this is not a reliable or recommended method of allergen verification. Adolescents were most likely to verify the food was allergen free by using a reliable strategy at home, school, and restaurants. Adolescents reported they engaged in these more reliable methods of allergen verification less than half the time when they ate in others’ homes or locations such as in the car/bus or sporting events. Thus, it appears that adolescents are routinely putting themselves at risk of allergic reaction by not using recommended methods. Our results are consistent with prior research that shows that most adolescents try to avoid allergens, but many do not ask about ingredients when outside the home.10,12,19
Notable study strengths include participant recruitment from a diverse pediatric allergy clinic; the majority of participants identified as non-White and were from a broad spectrum of socioeconomic backgrounds. An additional strength is that adolescents were the FAM-24 respondents, not parents. Given the age of the sample (10-14 years), parent report would likely be incomplete and inaccurate. Additional strengths include that the FAM-24 was based on a valid measure used among a type 1 diabetes population, and adolescents reported on the day prior to the interview, thus, reducing recall bias almost as much as possible. On the other hand, we were unable to extensively assess the reliability and validity of the FAM-24 without direct observation of the participants during a typical day and/or biomarkers of FA adherence, strategies that were used during development of the type 1 diabetes 24-hour recalls. We also did not administer the FAM-24 at multiple time points and were unable to assess the frequency of precautionary allergen labels on participants’ food. Furthermore, given the unpredictability and low frequency of allergic reactions, we did not capture behavior on days during which allergic reactions occurred. It is possible that behavior differed on days when participants experienced allergic reactions. Despite these limitations, we believe that this iteration of the FAM-24 provides a robust assessment of daily FA management behaviors.
Future Research
Use of the FAM-24 among a diverse sample has provided a rich overview of how adolescents manage their FAs. A new revised version of the FAM-24 will include further explication of how foods that are prepared at home are verified as allergen-free. Future studies should assess parent and child report independently, include multiple assessment time points, and further examine demographic, medical, and contextual factors that may affect FA management behavior. Furthermore, building a user-friendly digital platform to use the FAM-24 as an ecological momentary assessment tool would further reduce the possibility of recall bias and may present a viable method to assess precautionary allergy labeling because adolescents could complete the FAM-24 in the moment. Furthermore, this format would permit adolescents to complete the FAM-24 without an interviewer and would improve our ability to collect large quantities of data, which would increase the likelihood that we would capture data regarding behavior surrounding allergic reactions. Future applications of the FAM-24 may include use as a tool to assess change in FA behaviors over time. For example, it would be useful to document patterns of FA self-management across the developmental spectrum, to better understand when the ratio of parent-child FA management responsibility shifts as children get older. Further, although change in FA-related quality of life is a desirable target of psychosocial interventions, change in FA management behaviors that lead to a reduction in allergic reactions and healthcare utilization are equally important. The FAM-24 could also be used as a marker of behavior change pre-and post-interventions designed to improve FA self-management.
Conclusions
This first use of the FAM-24 to assess FA self-management behaviors among adolescents shows that they report varying behavior across regimen categories (EAI availability, allergen avoidance) and across meal/snack locations. Most concerning may be frequent reliance on experience with food in the past as a method of allergen avoidance. Clinicians may wish to query how patients make decisions about food safety and provide more education about appropriate methods of ascertaining the presence of allergens in their food. Additional work with the FAM-24 may further elucidate FA self-management behaviors and be key to tracking behavior change among adolescents and caregivers affected by FA.
Acknowledgments
Funding Source:
This project was funded by 5K23AI30184-02 (NIAID) awarded to Linda Herbert, PhD.
Abbreviations:
- (AAAAI)
American Academy of Allergy, Asthma, and Immunology
- (EAI)
epinephrine auto-injector
- (FA)
food allergy
- (FAM-24)
Food Allergy Management 24-Hour Recall
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None
Clinical Trial Registration: N/A
References
- 1.Marrs T, Lack G. Why do few food-allergic adolescents treat anaphylaxis with adrenaline? - Reviewing a pressing issue. Pediatr Allergy Immunol. 2013;24(3):222–229. doi: 10.1111/pai.12013 [DOI] [PubMed] [Google Scholar]
- 2.Trower A, Gettings S. Use of a food allergy care management pathway in adolescents. Nurs Child Young People. 2015;27(5):16–20. doi: 10.7748/ncyp.27.5.16.e582 [DOI] [PubMed] [Google Scholar]
- 3.Rona RJ, Keil T, Summers C, et al. The prevalence of food allergy: A meta-analysis. J Allergy Clin Immunol. 2007;120(3):638–646. doi: 10.1016/j.jaci.2007.05.026 [DOI] [PubMed] [Google Scholar]
- 4.Gupta RS, Warren CM, SMith BM, et al. The public health impact of parent-reported childhood food allergies in the United States. Pediatrics. 2018;142(6):e20181235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben-Shoshan M, Clarke AE. Anaphylaxis: Past, present and future. Allergy Eur J Allergy Clin Immunol. 2011;66(1):1–14. doi: 10.1111/j.1398-9995.2010.02422.x [DOI] [PubMed] [Google Scholar]
- 6.Bock SA, Muoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107(1):191–193. doi: 10.1067/mai.2001.112031 [DOI] [PubMed] [Google Scholar]
- 7.Fierstein JL, Brown D, Gupta R, Bilaver L. Understanding food related allergic reactions through a US National Patient Registry. J Allergy Clin Immunol Pract. Published online 2020. doi: 10.1016/j.jaip.2020.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenstock IM. Why people use health services. Milbank Mem Fund Q. 1966;44:94–127. [PubMed] [Google Scholar]
- 9.Monks H, Gowland MH, MacKenzie H, et al. How do teenagers manage their food allergies? Clin Exp Allergy. 2010;40(10):1533–1540. doi: 10.1111/j.1365-2222.2010.03586.x [DOI] [PubMed] [Google Scholar]
- 10.Sampson MA, Muñoz-Furlong A, Sicherer SH. Risk-taking and coping strategies of adolescents and young adults with food allergy. J Allergy Clin Immunol. 2006; 117(6): 1440–1445. doi: 10.1016/j.jaci.2006.03.009 [DOI] [PubMed] [Google Scholar]
- 11.Shah E, Pongracic J. Food-induced anaphylaxis: Who, what, why, and where? Pediatr Ann. 2008;37(8):536–541. [DOI] [PubMed] [Google Scholar]
- 12.Jones CJ, Llewellyn CD, Frew AJ, Du Toit G, Mukhopadhyay S, Smith H. Factors associated with good adherence to self-care behaviours amongst adolescents with food allergy. Pediatr Allergy Immunol. 2015;26(2): 111–118. doi: 10.1111/pai.12333 [DOI] [PubMed] [Google Scholar]
- 13.Gallagher M, Worth A, Cunningham-Burley S, Sheikh A. Epinephrine auto-injector use in adolescents at risk of anaphylaxis: A qualitative study in Scotland, UK. Clin Exp Allergy. 2011. ;41(6):869–877. doi: 10.1111/j.1365-2222.2011,03743.x [DOI] [PubMed] [Google Scholar]
- 14.Saleh-Langenberg J, Flokstra-de Blok BMJ, Goossens NJ, Kemna JC, van der Velde JL, Dubois AEJ. The compliance and burden of treatment with the epinephrine auto-injector in food-allergic adolescents. Pediatr Allergy Immunol. 2016;27(1):28–34.doi: 10.1111/pai.12458 [DOI] [PubMed] [Google Scholar]
- 15.Duncan SE, Annunziato RA. Barriers to self-management behaviors in college students with food allergies. J Am Coll Heal. 2018;66(5):331–339.doi: 10.1080/07448481.2018.1431898 [DOI] [PubMed] [Google Scholar]
- 16.NIAID-Sponsored Expert Panel, Boyce JA, Assa’ad A, et al. Guidelines for the diagnosis and management of food allergy in the United States: Report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 SUPPL.):301–402.doi: 10.1016/j.jaci.2010.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones CJ, Smith HE, Frew AJ, Toit G Du, Mukhopadhyay S, Llewellyn CD. Explaining adherence to self-care behaviours amongst adolescents with food allergy: A comparison of the health belief model and the common sense self-regulation model. Br J Health Psychol. 2014;19(1):65–82. doi: 10.1111/bjhp.12033 [DOI] [PubMed] [Google Scholar]
- 18.Robinson M, Koplin JJ, Field MJ, et al. Patterns of carriage of prescribed adrenaline autoinjectors in 10- to 14-year-old food-allergic students: A population-based study. J Allergy Clin Immunol Pract. 2019;7(2):437–443. doi: 10.1016/j.jaip.2018.06.025 [DOI] [PubMed] [Google Scholar]
- 19.Warren CM, Dyer AA, Otto AK, et al. Food allergy-related risk-taking and management behaviors among adolescents and young adults. J Allergy Clin Immunol Pract. 2017;5(2):381–390.el3. doi: 10.1016/j.jaip.2016.12.012 [DOI] [PubMed] [Google Scholar]
- 20.McLean RM, Farmer VL, Nettleton A, et al. Twenty-Four-Hour Diet recall and Diet records compared with 24-hour urinary excretion to predict an individual’s sodium consumption: A Systematic Review. J Clin Hypertens. 2018;20(10):1360–1376. doi: 10.1111/jch.13391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker JL, Ardouin S, Burrows T. The validity of dietary assessment methods to accurately measure energy intake in children and adolescents who are overweight or obese: A systematic review. Eur J Clin Nutr. 2018;72(2):185–197. doi: 10.1038/s41430-017-0029-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Igudesman D, Crandell J, Zhong VW, et al. Dietary intake on days with and without hypoglycemia in youth with type 1 diabetes: The Flexible Lifestyle Empowering Change trial. Pediatr Diabetes. 2020;(May):1–10. doi: 10.1111/pedi.13132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenwood DC, Hardie LJ, Frost GS, et al. Validation of the Oxford WebQ Online 24-Hour Dietary Questionnaire using biomarkers. Am J Epidemiol. 2019; 188(10):1858–1867. doi: 10.1093/aje/kwz165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Junge N, Migal K, Goldschmidt I, Baumann U. Transition after pediatric liver transplantation - Perceptions of adults, adolescents and parents. World J Gastroenterol. 2017;23(13):2365–2375. doi: 10.3748/wjg.v23.i13.2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson SB, Silverstein J, Rosenbloom A, Carter R, Cunningham W. Assessing daily management in childhood diabetes. Health Psychol. 1986;5(6):545–564. [DOI] [PubMed] [Google Scholar]
- 26.Reynolds LA, Johnson SB, Silverstein J. Assessing daily diabetes management by 24-hour recall interview: the validity of children’s reports. J Pediatr Psychol. 1990;15(4):493–509. doi: doi: 10.1093/jpepsy/15.4.493. [DOI] [PubMed] [Google Scholar]
- 27.Freund A, Johnson SB, Silverstein J, Thomas J. Assessing daily management of childhood diabetes using 24-hour recall interviews: reliability and stability. Heal Psychol. 1991;10(3):200–208. doi:doi: 10.1037//0278-6133.10.3.200. [DOI] [PubMed] [Google Scholar]
- 28.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]


