Figure 1. Opportunities for biomaterials to enhance engineered T cell therapies.
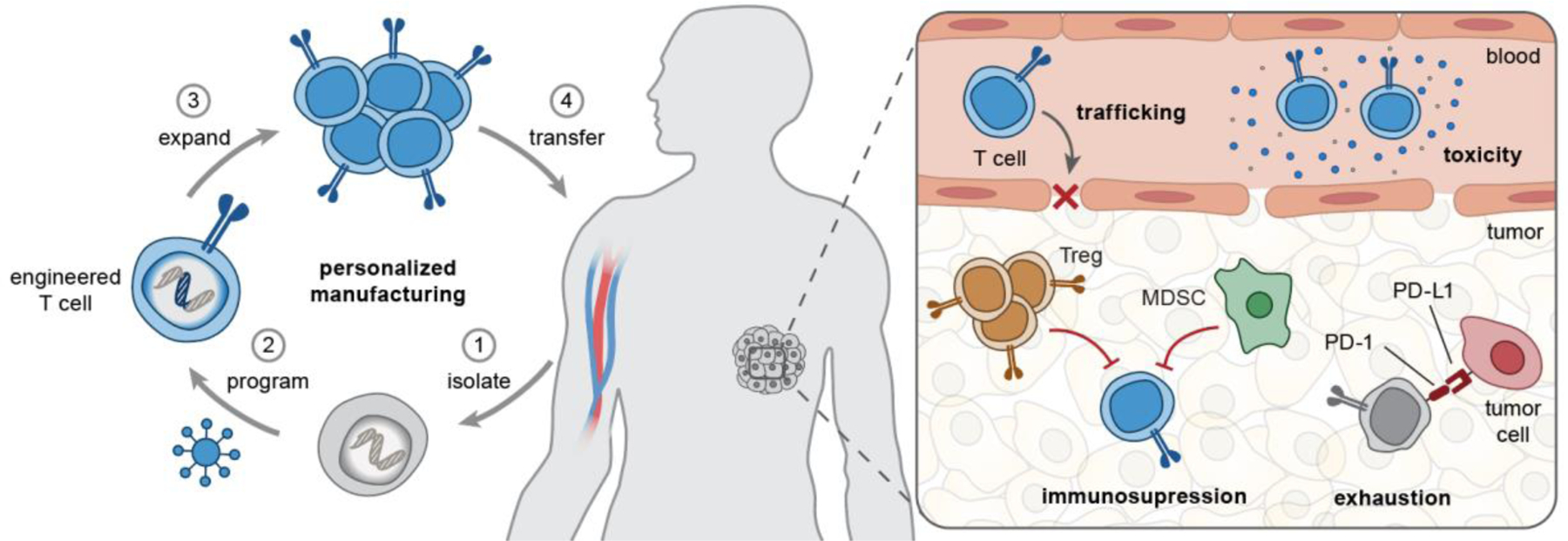
(Left) T cell manufacturing is a personalized, multi-step process that includes isolation of patient T cells, genetic programming using viral vectors, and ex vivo T cell expansion before autologous infusion. (Right) In vivo, engineered T cells need to overcome several challenges associated with T cell trafficking, tumor immunosuppression (e.g., by Treg, MDSCs), exhaustion by chronic antigen stimulation, and immune-related toxicities. MDSC, myeloid-derived suppressor cells; Treg, regulatory T cells; PD-1/PD-L1, programmed death-1/ligand-1; TME, tumor microenvironment.
