Figure 1: Full-length mouse ADAMTS7 purification and in vitro TSP1 cleavage.
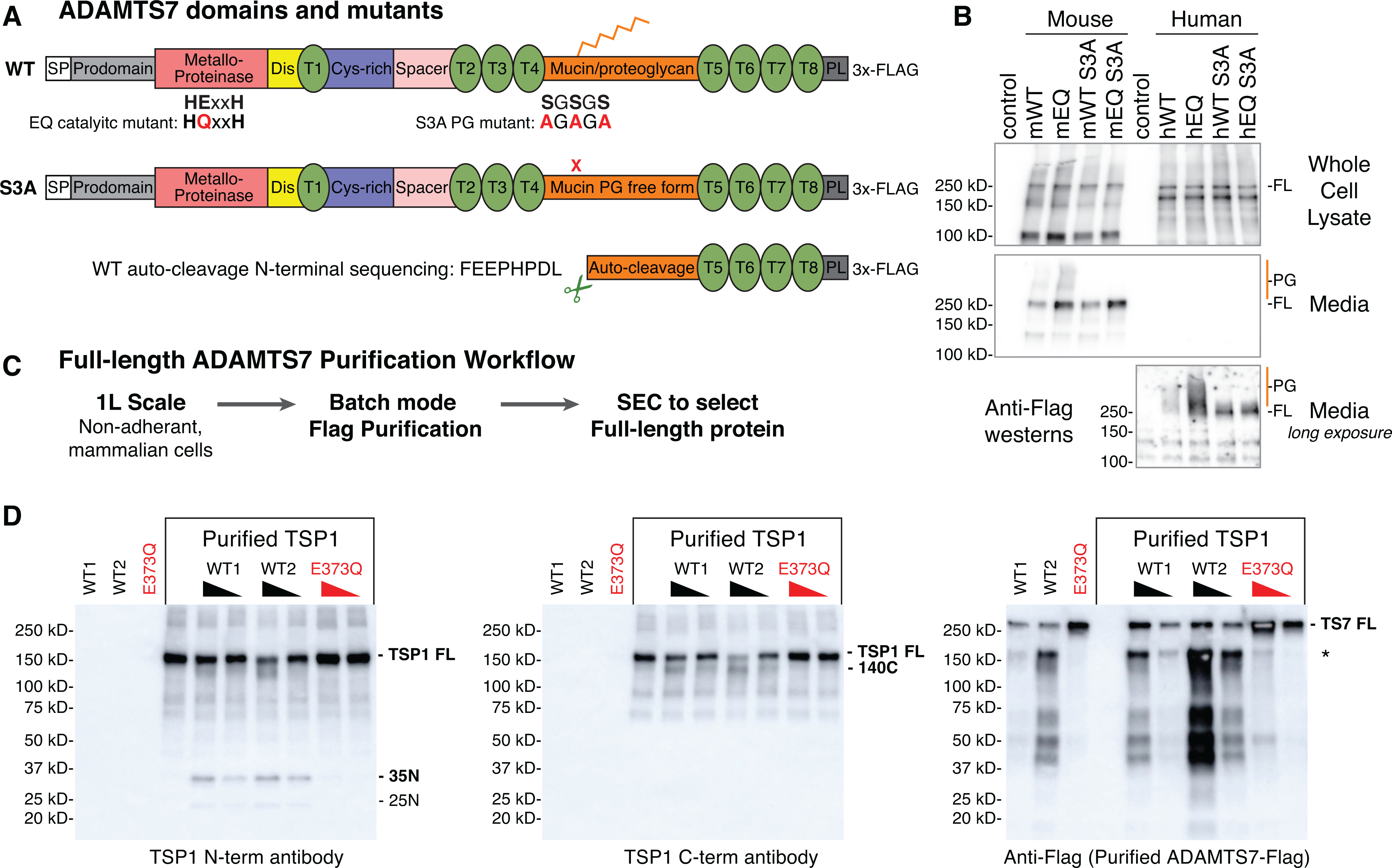
A, ADAMTS7 protein domains and locations of the glutamate to glutamine (EQ) catalytic mutant and serine to alanine (S3A) substitutions to prevent proteoglycan (PG) attachment. Abbreviated ADAMTS7 domains: signal peptide (SP), disintegrin (Dis), thrombospondin repeats (T), cysteine-rich (Cys-rich), protease and lacunin (PL). B, expression of full-length (FL) mouse and human ADAMTS7 3xFlag proteins in the whole cell lysate and secreted in the media under reducing conditions, detected by western blot using the M2-HRP antibody. High molecular weight proteoglycan (PG) species are marked in orange. A longer exposure of the conditioned media was required to visualize the secreted human ADAMTS7 proteins. C, full-length ADAMTS7 two step purification workflow used to purify WT S3A active enzyme and EQ S3A negative control protein. D, Thrombospondin1 in vitro cleavage by purified full-length mouse ADAMTS7 WT protein (* indicates co-purified auto-cleavage band). Western blots under reducing conditions to resolve proteolytic TSP1 bands generated by active ADAMTS7 WT purified enzyme. Presence of the E373Q catalytic mutation in the purified full-length mouse ADAMTS7 EQ protein ablated the catalytic activity.
