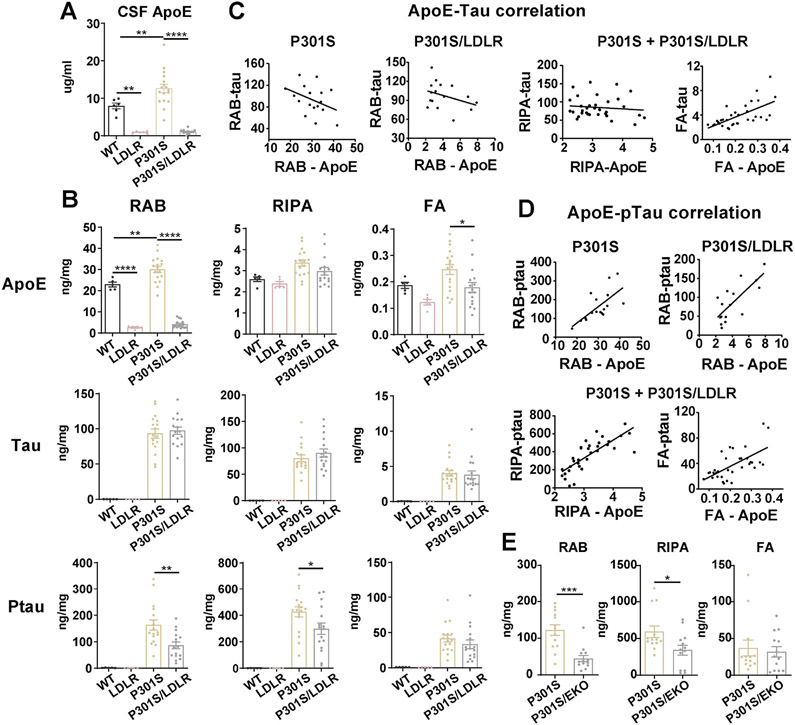
Figure 2. LDLR OX in P301S mice markedly reduces apoE and p-tau levels that highly correlate with each other.
(A) ApoE level in the CSF of 9-month WT, LDLR, P301S and P301S/LDLR mice measured by ELISA (n=6-17).
(B) ELISA measurement of apoE, human tau, and p-tau levels in RAB, RIPA and 70% FA fractions respectively for 9-month mouse posterior cortical lysates. Student’s t-test (two-sided) for human tau and ptau, no signal in WT and LDLR mice.
(C) Correlation between apoE and human tau level in RAB (non-significant), RIPA (no correlation) and FA (r2=0.4114, p<0.0001) fractions respectively in P301S and P301S/LDLR mice.
(D) Correlation between apoE level and p-tau level in RAB (P301S: r2=0.4649, p=0.0026; P301S/LDLR: r2=0.5690, p=0.0007), RIPA (r2=0.6124, p<0.0001), and FA (r2=0.3948, p<0.0001) fractions respectively in P301S and P301S/LDLR mice.
(E) ELISA measurement of p-tau levels in RAB, RIPA and 70% FA fractions of mouse brain lysates for a separate cohort of 9-month P301S and P301S/EKO mice. (n=13). Mann-Whitney test (two-sided).
Data expressed as mean ± SEM, One-way ANOVA with Tukey’s post hoc test, two-sided in (A) and (B), Pearson correlation analysis (two-sided) in (C) and (D). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.