Abstract
Introduction
Intravenous leiomyomatosis (ILV) is a rare pathology, part of leiomyoma beyond the uterus (LBU), characterized by benign smooth muscle cell tumor outside of the uterus and mainly affecting premenopausal woman with a medical history of leiomyoma or gynecologic surgical treatment. The treatment depends on the localization of the tumor, age of the patient, initial size, symptoms and the suitability for surgery but should always aims in toto surgical resection.
Case presentation
Retrospective case series and review of literature.
Clinical discussion
Symptoms presented by the patient were aspecific and only localized in the pelvic area. All cases were fortuitous histopathological diagnosis. No relapse was. Two out of 5 patients have pulmonary nodules, only one was biopsied and diagnosed with PBML (pulmonary benign metastasizing leiomyoma).
Conclusion
IVL and BML are rare disease that can co-exist. Because of tumoral hormonal receptors, hormonotherapy could be an optional treatment but to date no clear efficacy is demonstrated. In case of high recurrence risk such as voluminous initial mass, impairment of broad ligament, failure of total surgical resection, adjuvant hormonotherapy could be useful. Recurrence rate is about 16.6-30% and can occur even dozen years later and even after radical surgery, justifying a regular follow up.
Keywords: Leiomyomas, Leiomyomas beyond the uterus, Intravenous leiomyomatosis, Benign metastasizing leiomyomas, Surgery, Hormonotheray
Highlights
-
•
Leiomyomas beyond the uterus (LBU) is defined by benign smooth muscle cell tumor outside of the uterus. Sub classification of this entity are intravenous leiomyomatosis (IVL), benign metastasizing leiomyomas (BML), diffuse peritoneal leiomyomatosis (DPL), retroperitoneal leiomyomas and parasitic leiomyomas.
-
•
The differential diagnosis of IVL should include benign myoma, thrombus, leiomyosarcoma, soft tissue sarcoma, lymphoma, cardiac myxoma, tumor thrombosis of Wilms tumor or metastasis.
-
•
Treatment of IVL is usually surgery but there is no consensus about the the optimal approach. Adjuvant therapy sur as bilateral salpingoophorectomy followed by hormonothetherapy have to be balanced with their side effects.
-
•
Despite being histologically benign, BML shows the metastatic potential of LBU.
-
•
Because of a high rate of recurrence estimated of 16.6% to 30%, long term follow up is recommended even after radical surgery.
1. Background
Leiomyomas is one of the most common gynaecological benign neoplasia with estimated incidence of 5.4% to 77% of women of reproductive age and characterized by benign smooth muscle neoplasms inside of the uterus [1]. Leiomyomas beyond the uterus (LBU) is defined by benign smooth muscle cell tumor outside of the uterus. Sub classification of this entity is intravenous leiomyomatosis (IVL), benign metastasizing leiomyomas (BML), diffuse peritoneal leiomyomatosis (DPL), retroperitoneal leiomyomas and parasitic leiomyomas [2]. IVL is a rare pathology, first described in 1896 by Birsch-Hirschfeld [3], that affects only woman, and characterized by benign smooth muscle growing within vascular spaces of the venous system [4], [5], [6]. Incidence of this disease is about 0.25% to 0.40% of patients who present uterine fibroma [7]. Until now, approximately 300 cases have been reported in the literature.
We hereby present a 20 years retrospective single institution case series of five patients and a review of actual medical recommendations (Table 1).
Table 1.
| Case | Age (yrs) | Menopausal status | Clinical presentation | G & P | Surgical procedure | Tumoral size (cm) | Weight (g) | Histological findings | Staging | Follow up (mo) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 52 | Yes | Menorrhagia | G1P0 | TLH + BSO | 3 | 160 | Actin, desmin, caldesmon + Factor VII, CD31 + |
1 | 5 |
| 2 | 50 | Yes | Pelvic mass | U | TLH + BSO | 7 | 300 | Actin, desmin, caldesmon + | 1 | NED (12) |
| 3 | 35 | No | Pelvic mass rapidly growing | G1P1 | TAH + BS | 10 | 752 | CD 10 negative, caldesmon, actin +, progesterone 100%, estrogen 80% | 1 | NED (48) |
| 4 | 46 | No | Pelvic pain | G3P2 | TLH + BS | 10 | 680 | CD 31, CD34 + | 1 | LFU |
| 5 | 43 | No | Hypermenorrhea | G53P | STLH + BS | 5 | 340 | CD 10 negative, CD 34 et 31 + | 1 | NED (72) |
Abbreviations: BML, benign metastasizing leiomyomas; BS, bilateral salpingectomy; BSO, bilateral salpingo-oophorectomy; LFU, lost to follow up; mo, months; NED, no evidence of disease; TAH, total abdominal hysterectomy; STLH, subtotal laparoscopic hysterectomy; TLH, total laparoscopic hysterectomy, U, unknown, yrs.; years.
2. Methods
Respecting the SCARE 2020 criteria we collected the following data of five patients [8].
3. Case 1
A 52 years old nulliparous Caucasian woman known to be overweight (BMI 32), with myomatous uterus, presented to her gynecologist with menometrorragia.
Endovaginal echocardiography showed a fundal myoma FIGO 1 of 7x9cm. The thin prep cytologic test and endometrial sampling analysis were normal.
The patient underwent a total hysterectomy with adnexectomy by laparoscopy with no operative complication. The macroscopic operative status was normal. Pathological examination concludes for squamous metaplasia of the endocervical mucosa, leiomyoma and intravenous leiomyomatosis. Microscopic examination revealed no mitosis, no atypia nor necrosis. Immunofixation showed reaction for actin, demine and caldesmon and weak reaction with CD10.
Because of this fortuitous diagnosis, she underwent a cardiac echocardiography which showed no occlusive mass form the inferior vena cava or cardiac dysfunction. A thoracic Computed Tomography (CT) scan pointed well-circumscribed bilateral nodular lung lesions from 4 to 8 mm in the antero-superior and inferior pulmonary lobes (Fig. 1). The patient declaimed no pulmonary symptoms. Because of this highlighting, Positron Emission Computed Tomography (PET-CT) was organized but revealed no metabolic translation of the pulmonary nodules but one posterieur hypercaptive (SUV max 2,2) centrimetric subcutanous nodule which showed, after cutaneous biopsy, no cellular atypia and was probably lipoma. She underwent a laparoscopic wedge resection of one pulmonary lesion with prior radiological tracking wire. Anatomo-pathological analysis of the pulmonary tissue revealed an intraparenchymal well-defined mass, pushing back the airways tissue to periphery. Final diagnosis is pelvic low grade IVL with pulmonary BML (PBML). Before thoracic surgery, she gets infected by SARSCoV-2 and developed pneumonia with hypoxemic acute respiratory failure but with fast rehabilitation and only needed for oxygen and corticosteroid therapy.
Fig. 1.

CT scan showing well-circumscribed millimeter nodular lung lesion of the left antero-superior pulmonary lobe.
4. Case 2
A 50 years old menopausal woman presented a voluminous uterus with preoperative suspicion of endometrial cancer. She underwent a laparoscopic hysterectomy with adnexectomy without any complication. Macroscopic evaluation of the uterus, fallopian tubes and ovaries was normal. Pathological examination of the uterus showed a quiet well-defined lobulated tumor of 7 cm length inside of the myometrium. The microscopic examination revealed a leiomyomatous tumor with well-developed vascular network and vascular invasion outside of the mass by invasive cords rowing inside of the venous lumen, in direction of the right parametrium. Both adnexa were normal.
No other imaging was organized and the patient was lost to follow up after one year.
5. Case report 3
A 38 years old 1G1P obese (BMI 38) Caucasian woman developed a rapidly-growing uterine mass of 10x8x8cm. The MRI confirmed the uterine origin and objectivated submucosal and subserous development from the mass. Suspecting a sarcoma she underwent an abdominal hysterectomy with bilateral salpingectomy. The post-operative pathological examination denied this diagnosis and revealed IVL.
Immunofixation was negative for CD 10, caldesmon and actin positive. Progesterone and estrogen receptors were highly positive. The surgical margins were free from tumoral invasion. She underwent a complete check up with echocardiography and thoracic CT which found three sub pleural nodules in the apical segment and the inferior part of the laterobasal segment, measuring from 6 to 8 mm. She never underwent pulmonary biopsy, and the control 3 years later showed no progression of those nodules without any hormonal treatment and the patient never complained about respiratory symptoms.
6. Case 4
A 46 years old 3G2P woman with amenorrhea due to intrauterine progestogen device presented with pelvic pain. The endovaginal ultrasound concludes to a myomatous uterus. She had undergone a total laparoscopic hysterectomy with bilateral salpingectomy. Uterus was about 680 g and showed a voluminous whitish fasciculate in appearance mass of 10x8x8cm. Pathological examination concluded with intravascular leiomyomatosis. A thoracic CT showed no other lesions and she was lost to follow up.
7. Case 5
A 43 years old woman suffered from hypermenorrhea. Multimyomatous uterus was diagnosed with endovaginal ultrasound. Because of failure of a hormonal medical treatment, she underwent a subtotal hysterectomy by laparoscopy with intra-abdominal morcellation. The post-operative pathological examination showed intravascular leiomyomatosis and because of the grinding of the surgical specimen, it was not possible for the pathologists to pronounce if the excision was in healthy tissue.
Six years after diagnosis, she presented no sign of recurrence without any other treatment.
8. Results
Median age at diagnosis of our series is 45.2 years. Symptoms presented by the patient were aspecific (abnormal bleeding and/or pelvic pain) and only localized in the pelvic area. The median uterus weight was 446 g (from 160 g to 752 g). One patient presented a rapidly growing uterine mass suspected for sarcoma.
All cases were fortuitous histopathological diagnosis.
No relapse was identified but two of our patients were lost to follow up and one is a very recent case. Two out of 5 patients have pulmonary nodules, only one was biopsied and diagnosed with PBML.
Patient with PBML get infected with SARSCoV-2 in Mai 2021. She required a short hospitalization 10 days after the first positive Polymerase Chain Reaction (PCR) test. No other case of PBML patient with SARSCoV-2 infection is actually described.
9. IVL: review of literature
9.1. Pathogenesis
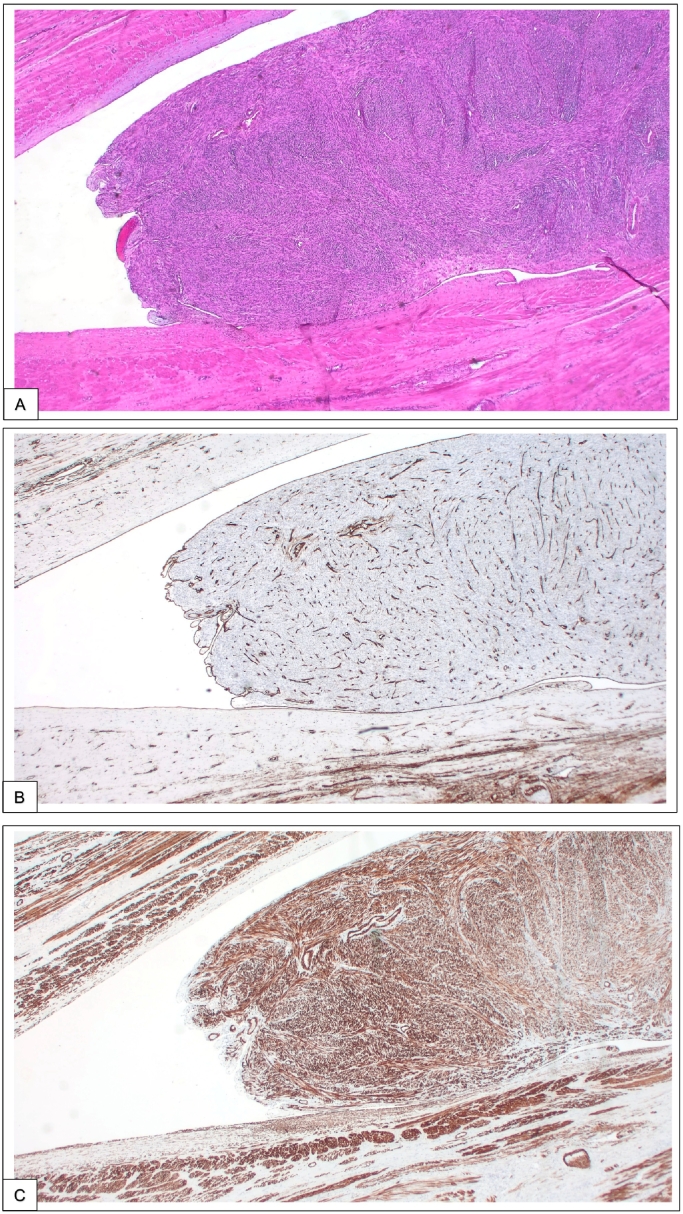
Etiology of IVL remains unclear. Two theories postulates for the pathological development: Steinmetz et al. thought that IVL growth form a preexisting uterine leiomyoma which grows and progressively invade vascular lumen [9]. Knauer pretend that IVL could develop by metaplasia from the smooth muscle cells from uterine venular walls [10]. The second hypothesis seems unlikely for Ma et al. because of the absence or rarity of endothelial marker such as CD10, CD 31 or CD34 [7]. However those markers were positive for some of our patients (Fig. 2).
Fig. 2.
A, Intravascular tumor almost completely filling the lumen of a small vein with a dense cellular proliferation of uniform spindle-shaped smooth muscle cells (hematoxylin and eosin (H&E), magnification × 50). B, Immunohistochemical brown staining for factor VIII, an endothelial marker, showing an endothelial recovering of the intravascular leiomyoma (magnification × 50). C, Immunohistochemical brown staining for caldesmon, a marker of smooth muscle, showing an intense staining of the intravascular leiomyoma as well as the muscular wall of the vein (magnification × 50). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
9.2. Histological characters and genetics dysregulation
Histologic description corresponds to typical uterine myoma with immuno-histological reaction for actine, desmin, caldesmon which are muscular markers. Cells exhibit positivity for estrogen or progesterone receptors and mitosis rate is rather low [11].
Some molecular difference are observed in between uterine myoma and IVL such as dysregulation of HOXA13 - a specific gene for embryonic development and cell differentiation [12], or MED12 mutation, which has a critical and central role in RNA polymerase II transcription [13]. A higher rate of chromosomal aberration is observed in IVL despite the fact that both share some molecular characteristics. High-mobility group AT-hook 2 (HMGA 2), a protein acting for mesenchymal differentiation, is suspected in pathogenesis of IVL transformation [14].
Sampling is very important for IVL diagnosis especially to observe not only the tumor but also the smooth muscle tissue surrounding for exact diagnosis [15]. Thanks to the vigilance and the actual attention paid by the pathologist to this diagnosis, IVL is no more underdiagnosed.
9.3. Clinical manifestations
Although IVL is a histologically benign condition, it might have a malignant behavior and typically spreads unilaterally by two possible venous ways from the uterus: through the uterine veins to the iliac veins and then to inferior vena cava (IVC) or from the ovarian veins directly reaching the IVC. IVL never shows any vascular adhesion [6], [16] and obstruction of the vascular lumen is never complete [17]. Depending on the localization of the invasion, symptoms are non-specific and can mimic uterine myoma or be as worth as chest pain, dyspnea or cardiac arrest when the tumor invades the cardiac chambers [11]. Risks factors to develop IVL are uterine myoma, previous surgical procedure of the uterus such as hysterectomy or myomectomy and a prior IVL diagnosis [18].
9.4. Imagery
Diagnostic imagery for IVL or follow up is MRI, CT scanner, or echocardiography [19], [20], [21]. Echography is useful for IVL with cardiac extension with visualization of intravascular long serpentines, such as multiple stripe-like hyperechogenic lines, filled with colored blood flow [22]. If extension of IVL is restricted inside of the small veins of the uterus, detection is very challenging [15]. It seems that CT is superiors to MRI or US in mapping the full path of tumor extension [19].
Classified in 4 level of extension by MRI or CT, staging is very useful for the pre-operative assessment and organization of a multidisciplinary approach (Fig. 3) [7], [23]. In this case series, we only described patients with stage I. PET CT imagery for IVL diagnosis point low metabolic activity in contrast to malignant lesions but shows no other advantage [24].
Fig. 3.

Stage of intravenous leiomyomatosis was categorized into 4 stages helping surgical management. Stage I corresponds to a penetrative tumor towards the uterine venous wall, but confines to the pelvic cavity. Where tumor has extends into the abdominal cavity, but without reaching the renal vein, categorization is a stage II. If the tumor reaches the renal vein and IVC, or the right atrium, but without reaching the pulmonary arteries, patients were categorizes as stage III. Stage IV corresponds to metastases or invasion of the pulmonary arteries.
The differential diagnosis of IVL should include benign myoma, thrombus, leiomyosarcoma, soft tissue sarcoma, lymphoma, cardiac myxoma, tumor thrombosis of Wilms tumor or metastasis [2], [25], [26], [27].
9.5. Treatment
Treatment of IVL is usually surgery [28]. There is no consensus for the optimal approach for IVL resection [29] but current recommendations for any stages are complete surgical resection such as total hysterectomy. A few cases with simple myomectomy are reported, especially young patients with desire of fertility preserving. Choice of surgical technics (laparoscopy versus laparotomy) seems to have no impact on the recurrence rate and varies among surgeons and mainly depends on the tumors size and the health condition of the patient that impact her tolerance to surgery [7], [11]. Few studies propose as gold standard adjuvant therapy to proceed to bilateral salpingoophorectomy followed by hormonothetherapy [30].
For more advance stages, depending on the tumoral vascular extension and the suitability for the patient to surgery, early treatment is recommended with a single or double procedure by multidisciplinary team [31], [32]. If the tumor reaches cardiac level, sternotomy is followed by abdominal resection by laparotomy. Recent study shows that thoracotomy could be avoided by gently pulling the tumor from the abdominal level [28]. Nowadays one-stage approach are more frequently proposed because of increased knowledge of this disease [30], [33]. Most of the one-stage procedures are performed with cardiopulmonary bypass and total circulatory arrest but it is possible to proceed with heart beating surgery because of the absence of adhesion to the IVC or the heart [34], [35]. One stage operation avoid the risk of tumor embolism in between the two operation, tumoral progression or repeated surgery [28], [29].
Difficulty of surgery depends more on the degree of tumors invasion and deformation of the vessel walls, than with its length. Complications of major surgical operations are death, hemorrhage due to peri-tumoral hypervascularization, infections, embolism and the usual post laparotomy complications [31], [36], [37]. To prevent hemorrhage, preoperative embolization of the uterine arteries or uterine arteries ligation may be appropriate [28].
9.6. Quick update on BML
100 cases have been reported [23] with first reported case in 1939 [38]. Despite being also histologically benign, BML shows the metastatic potential of LBU. Reported metastasizing sites are spine, skull, rib, vertebra, parametria, appendix, lymph nodes, retroperitoneum and most of time; lungs [23], [24], [39], [40].
Most accepted hypothesis for PBML is a cell dislodging from the uterine wall during myomectomy or hysterectomy [23], but exact etiology still also unclear. Another hypothesis is that PBML metastasizes because hits low grade malignant power, considering PBML as a differentiated leiomyosarcoma. Third theory states that PBML is neither more a smooth muscle hamartoma nor a metastasis because of its multiple origins sometimes without uterine fibroma [24].
Because of the small interval between IVL and PBML diagnosis, our first case proves that no previous surgical intervention is required for developing BML which was already observed in a small case series [18]. Metastatic potential of LBU is probably inherent at any stage of this disease. A recent publication suggests that the type of surgery does not determine the risk of future incidence [18]. Mean time from primary surgery to BLM diagnosis is often longer with a median of 8.8 years and most of time diagnosis is only made after patients reporting pulmonary symptoms such as couch, dyspnea, chest pain, or even pneumothorax rather than routine imaging monitoring after BML diagnosis [18]. Rarely, PBML causes respiratory failure [41].
10. Discussion
IVL recurrence rate is estimated of 16.6% to 30% [5], [42], [43], [44]. A few risk factors are nowadays identified such as initial voluminous mass (>7 cm), impairment of broad ligament or failure of total surgical resection [45]. No recurrence was observed in our study. Literature reviews contradict each other if young patient presents a higher risk of recurrence [46], [47] but IVL is more frequent in premenopausal woman.
Because of estrogen and progesterone receptors, tumoral growth seems to be related to the hormonal level [47] and exogenous estrogen must be avoid [48]. To date regarding treatment for IVL, no clear efficacy was demonstrated for adjuvant hormonal treatment such as gonadotropin-releasing agonist, aromatase inhibitor or selective estrogen receptor modulators (SERM). Hormonal medication might be considered in case of high risk of recurrence such as a big initial tumors size or incomplete surgical resection. Some study recommend the use hormonal therapy, especially if the patient is premenopausal, in case of recurrence or in order to reduce the tumor size if the patient reclines surgery [49], [50], [51], [52], [53], [54], [55], [56], [57], but the side effects of premature menopause have to be balanced with the eventual benefices of this medication [58]. A case with increase of tumor burden was described after Tamoxifen treatment emphasizing the necessity to consider carefully the indication of hormonal therapy [59]. Radiotherapy at low doses is described in patients at high risk of recurrence [60].
Zhang et al. published in 2020 a case of IVL stage 3 controlled with Rapamycin medication (mTOR inhibitor. This could represent a potentially new treatment to reduce the tumor size before surgery or for inoperable patients [61].
No standard guidelines exist for PBML. Medical follow up for asymptomatic patients is long term observation and regular imaging examinations. PBML lesions are most of time slow growing tumors with no clinical repercussions. However, hormonal changes affect its growth and vigilance during menopause or during pregnancy is recommended. If patient reports clinical manifestations, antiestrogen therapy, gonadotropin releasing hormone agonist, SERM and aromatase inhibitor can be introduced or oophorectomy organized. When compressive symptoms occur, and no response to medical treatment is observed, surgical resection of PBML can be considered [62]. In our series 2 patients presented suspicion of PMBL without any pulmonary symptoms with one confirmed case. Even with asymptomatic patients, a complete imagery checkup, such as cardiac echography and thoraco abdominal CT scan should be organized after LBU diagnosis.
11. Conclusion
ILV is a rare disease, which can have significant consequences. Because of its rarity, screening for IVL before routine myoma surgery is not indicated and conducting randomized controlled trial is difficult [18].
IVL should be suspected before gynaecological surgery in case of giant pelvic mass, a broad ligament myoma [15] or with imagery with long serpentine that looks like “snakeheads” or “walsking stick heads”. Surgery should always aim for complete resection in order to decrease recurrence. When IVL patients present with multiple pulmonary nodules, BML diagnosis should be consider in order to avoid excessive treatment due to misdiagnosis as a malignant tumor [46]. Indication for hormonal therapy or bilateral salpingoophorectomy have to be balanced with theirs side effects and have to be consider within the overall clinical context [12]. In contrary of IVL, PBML require most of time only long term imaging follow up, without surgical indication.
Individual treatment strategy should be considered for each patient depending on the tumors location, the hormonal status, the age of the patient and the initial tumors size. We recommend a regular follow up after radical surgery and patient education about relapse symptoms as recurrence can occur even dozen of years later [48].
Funding
There was no founding for this case report.
All the authors worked on their free time about this paper.
Ethical approval
This is a case report about 5 patients between 2000 and 2020.
This is a retrospective study based on pathological reports. Our institution exempted us to submit the case from an ethnical approval.
Consent
And there is no abbreviation of the name of the patient of any way to recognize her.
The authors have obtained oral informed consent from the patient.
Registration of research studies
-
1.
Name of the registry: Research Registry
-
2.
Unique Identifying number or registration ID: research registry 6890
-
3.
Hyperlink to the registration (must be publicly accessible): https://www.researchregistry.com/browse-the-registry
Guarantor
Dr Mathey Marie-Pierre
CRediT authorship contribution statement
Marie-Pierre Mathey: carried out the structure of the article, the literature review and corrected the final version
Daniel Huber followed the patient, corrected several times and approved the final version.
Declaration of competing interest
There was no personal or professional interest by writing about this case.
The authors declare any conflict of interest in this article.
References
- 1.Stewart E.A., Cookson C.L., Gandolfo R.A., Schulze-Rath R. Epidemiology of uterine fibroids: a systematic review. BJOG. 2017 Sep;124(10):1501–1512. doi: 10.1111/1471-0528.14640. [DOI] [PubMed] [Google Scholar]
- 2.Fasih N., Prasad Shanbhogue A.K., Macdonald D.B., Fraser-Hill M.A., Papadatos D., Kielar A.Z., Doherty G.P., Walsh C., McInnes M., Atri M. Leiomyomas beyond the uterus: unusual locations, rare manifestations. Radiographics. 2008 Nov-Dec;28(7):1931–1948. doi: 10.1148/rg.287085095. [DOI] [PubMed] [Google Scholar]
- 3.Birsch-Hirschfeld F.V. Lehrbuch der Pathologischen Anatomie. 5th edn. FCW Vogel; Leipzig: 1896. pp. 226–258. [Google Scholar]
- 4.Valdés Devesa V., Conley C.R., Stone W.M., Collins J.M., Magrina J.F. Update on intravenous leiomyomatosis: report of five patients and literature review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013 Dec;171(2):209–213. doi: 10.1016/j.ejogrb.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 5.Tang L., Lu B. Intravenous leiomyomatosis of the uterus: a clinicopathologic analysis of 13 cases with an emphasis on histogenesis. Pathol. Res. Pract. 2018 Jun;214(6):871–875. doi: 10.1016/j.prp.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Declas E., Lucot J.P. La léiomyomatose extra-utérine: revue de la littérature [Extra uterine leiomyomatosis: review of the literature] Gynecol. Obstet. Fertil. Senol. 2019 Jul-Aug;47(7–8):582–590. doi: 10.1016/j.gofs.2019.06.010. French. Epub 2019 Jun 27. [DOI] [PubMed] [Google Scholar]
- 7.Ma G., Miao Q., Liu X., Zhang C., Liu J., Zheng Y., Shao J., Cheng N., Du S., Hu Z., Ren Z., Sun L. Different surgical strategies of patients with intravenous leiomyomatosis. Medicine (Baltimore) 2016 Sep;95(37) doi: 10.1097/MD.0000000000004902. PMID: 27631266; PMCID: PMC5402609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.SCARE Group. Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A. The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020 Dec;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. Epub 2020 Nov 9. [DOI] [PubMed] [Google Scholar]
- 9.Steinmetz O.K., Bedard P., Prefontaine M.E., Bourke M., Barber G.G. Uterine tumor in the heart: intravenous leiomyomatosis. Surgery. 1996 Feb;119(2):226–229. doi: 10.1016/s0039-6060(96)80174-7. [DOI] [PubMed] [Google Scholar]
- 10.Knauer E. Beitrag zur anatomie der uterusmyome. Beitr Geburtshilfe Gynaekol. 1903;1:695–735. [Google Scholar]
- 11.Li B., Chen X., Chu Y.D., Li R.Y., Li W.D., Ni Y.M. Intracardiac leiomyomatosis: a comprehensive analysis of 194 cases. Interact. Cardiovasc. Thorac. Surg. 2013 Jul;17(1):132–138. doi: 10.1093/icvts/ivt117. Epub 2013 Apr 5. PMID: 23563052; PMCID: PMC3686387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X., Wu L., Xu R., Zhu C., Ma G., Zhang C., Liu X., Zhao H., Miao Q. Identification of the molecular relationship between intravenous leiomyomatosis and uterine myoma using RNA sequencing. Sci. Rep. 2019 Feb 5;9(1):1442. doi: 10.1038/s41598-018-37452-3. PMID: 30723247; PMCID: PMC6363745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L., Hu S., Xin F., Zhao H., Li G., Ran W., Xing X., Wang J. MED12 exon 2 mutation is uncommon in intravenous leiomyomatosis: clinicopathologic features and molecular study. Hum. Pathol. 2020 May;99:36–42. doi: 10.1016/j.humpath.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Ordulu Z., Nucci M.R., Dal Cin P., Hollowell M.L., Otis C.N., Hornick J.L., Park P.J., Kim T.M., Quade B.J., Morton C.C. Intravenous leiomyomatosis: an unusual intermediate between benign and malignant uterine smooth muscle tumors. Mod. Pathol. 2016 May;29(5):500–510. doi: 10.1038/modpathol.2016.36. Epub 2016 Feb 19. PMID: 26892441; PMCID: PMC5891726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du J., Zhao X., Guo D., Li H., Sun B. Intravenous leiomyomatosis of the uterus: a clinicopathologic study of 18 cases, with emphasis on early diagnosis and appropriate treatment strategies. Hum. Pathol. 2011 Sep;42(9):1240–1246. doi: 10.1016/j.humpath.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Yano M., Katoh T., Nakajima Y., Iwanaga S., Kin R., Kozawa E., Yasuda M. Uterine intravenous leiomyomatosis with an isolated large metastasis to the right atrium: a case report. Diagn. Pathol. 2020 Jan 11;15(1):4. doi: 10.1186/s13000-019-0913-2. PMID: 31926551; PMCID: PMC6954539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dankoro A., Foucher E., Grossin M., Mandelbrot L. Léiomyomatose intravasculaire de l'utérus [Intravascular leiomyomatosis of the uterus. Two cases and review of the literature] J. Gynecol. Obstet. Biol. Reprod. (Paris) 2004 Dec;33(8):753–757. doi: 10.1016/s0368-2315(04)96638-0. French. [DOI] [PubMed] [Google Scholar]
- 18.Barnas E., Ksiazek M., Ras R., Skret A., Skret-Magierlo J., Dmoch-Gajzlerska E. Benign metastasizing leiomyoma: a review of current literature in respect to the time and type of previous gynecological surgery. PLoS One. 2017 Apr 20;12(4) doi: 10.1371/journal.pone.0175875. PMID: 28426767; PMCID: PMC5398563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gui T., Qian Q., Cao D., Yang J., Peng P., Shen K. Computerized tomography angiography in preoperative assessment of intravenous leiomyomatosis extending to inferior vena cava and heart. BMC Cancer. 2016 Feb;8(16):73. doi: 10.1186/s12885-016-2112-9. PMID: 26858203; PMCID: PMC4746779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang L.Q., Zhang B., Liu B.G., Liu F.H. Diagnosis of intravenous leiomyomatosis extending to heart with emphasis on magnetic resonance imaging. Chin. Med. J. 2012 Jan;125(1):33–37. [PubMed] [Google Scholar]
- 21.Ge Z., Wang Y., Qi Z., Zhang Q., Jin J., Li J. Ultrasound appearance of intravenous leiomyomatosis: a case report. Medicine (Baltimore) 2019 Aug;98(35) doi: 10.1097/MD.0000000000016913. PMID: 31464926; PMCID: PMC6736113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li R., Shen Y., Sun Y., Zhang C., Yang Y., Yang J., Su R., Jiang B. Intravenous leiomyomatosis with intracardiac extension: echocardiographic study and literature review. Tex. Heart Inst. J. 2014 Oct 1;41(5):502–506. doi: 10.14503/THIJ-13-3533. PMID: 25425982; PMCID: PMC4189351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan R., Feng F., Yang H., Xu K., Li S., You Y., Wan X., Zhu L. Pulmonary benign metastasizing leiomyomas: a case series of 23 patients at a single facility. BMC Pulm. Med. 2020 Nov 10;20(1):292. doi: 10.1186/s12890-020-01330-4. PMID: 33172427; PMCID: PMC7653756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai H.Y., Guo S.L., Shen J., Yang L. Pulmonary benign metastasizing leiomyoma: a case report and review of the literature. World J. Clin. Cases. 2020 Jul 26;8(14):3082–3089. doi: 10.12998/wjcc.v8.i14.3082. PMID: 32775390; PMCID: PMC7385613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmed M., Zangos S., Bechstein W.O., Vogl T.J. Intravenous leiomyomatosis. Eur. Radiol. 2004 Jul;14(7):1316–1317. doi: 10.1007/s00330-003-2186-z. [DOI] [PubMed] [Google Scholar]
- 26.Fang H., You Y., Cai F., Yang Y., Yang C., Lv P. Intravenous leiomyomatosis of the subclavian vein. J Vasc Surg Venous Lymphat Disord. 2017 Mar;5(2):254–256. doi: 10.1016/j.jvsv.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Wu Y.H., Lee Y.T., Lee C.I., Tzeng Y.H., Wei J. Nonthrombotic pulmonary embolism caused by intravenous leiomyomatosis: a case report. Medicine (Baltimore) 2019 Jan;98(3) doi: 10.1097/MD.0000000000014118. PMID: 30653137; PMCID: PMC6370129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marrone G., Crinò F., Morsolini M., Caruso S., Miraglia R. Multidisciplinary approach in the management of uterine intravenous leiomyomatosis with intracardiac extension: case report and review of literature. J Radiol Case Rep. 2019 Jul 31;13(7):1–13. doi: 10.3941/jrcr.v13i7.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price J.D., Anagnostopoulos C., Benvenisty A., Kothuru R.K., Balaram S.K. Intracardiac extension of intravenous leiomyomatosis. Ann. Thorac. Surg. 2017 Feb;103(2):e145–e147. doi: 10.1016/j.athoracsur.2016.07.037. [DOI] [PubMed] [Google Scholar]
- 30.Lou Y.F., Shi X.P., Song Z.Z. Intravenous leiomyomatosis of the uterus with extension to the right heart. Cardiovasc. Ultrasound. 2011 Sep 24;9:25. doi: 10.1186/1476-7120-9-25. PMID: 21943238; PMCID: PMC3192729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo G., Pan H., Bi J., Luo Y., Zhu J., Feng Z., Fan H., Zhang Y., Dai X. Surgical treatment of intravenous leiomyomatosis involving the right heart: a case series. J. Int. Med. Res. 2019 Jul;47(7):3465–3474. doi: 10.1177/0300060519858021. Epub 2019 Jul 7. PMID: 31280644; PMCID: PMC6683876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Topcuoglu M.S., Yaliniz H., Poyrazoglu H., Tokcan A., Demir S.C., Bozkurt A., Zeren H. Intravenous leiomyomatosis extending into the right ventricle after subtotal hysterectomy. Ann. Thorac. Surg. 2004 Jul;78(1):330–332. doi: 10.1016/S0003-4975(03)01371-7. [DOI] [PubMed] [Google Scholar]
- 33.Su Q., Zhang X., Zhang H., Liu Y., Dong Z., Li G., Ding X., Liu Y., Jiang J. Intravenous leiomyomatosis of the uterus: a retrospective single-center study in 14 cases. Biomed. Res. Int. 2020 Feb;14(2020) doi: 10.1155/2020/9758302. PMID: 32337287; PMCID: PMC7155762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li B., Li R.Y., Chen X., Xu L.J., You Q.H., Ni Y.M., Li W.D. One-stage complete removal of intracardiac leiomyomatosis without cardiac arrest. Thorac. Cardiovasc. Surg. 2013 Jan;61(1):88–90. doi: 10.1055/s-0032-1324712. [DOI] [PubMed] [Google Scholar]
- 35.Kocica M.J., Vranes M.R., Kostic D., Kovacevic-Kostic N., Lackovic V., Bozic-Mihajlovic V., Velinovic M.M., Mikic ADj Dimitrijevic-Kalezic N. Intravenous leiomyomatosis with extension to the heart: rare or underestimated? J. Thorac. Cardiovasc. Surg. 2005 Dec;130(6):1724–1726. doi: 10.1016/j.jtcvs.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 36.Kong L.Y., Chen L.L., Xiang W., Liu F. Intravenous leiomyomatosis with paradoxical embolism: unusual presentation of uterine leiomyoma. Circ. Cardiovasc. Imaging. 2020 Jan;13(1) doi: 10.1161/CIRCIMAGING.119.009930. [DOI] [PubMed] [Google Scholar]
- 37.Corbett G.A., O’Gorman C., Kamran W. Intravenous leiomyomatosis: the first reported case of intraoperative intracaval embolisation of tumour to the right atrium. BMJ Case Rep. 2020 Mar 12;13(3) doi: 10.1136/bcr-2019-233341. PMID: 32169987; PMCID: PMC7069302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steiner P.E. Metastasizing fibroleiomyoma of the uterus: report of a case and review of the literature. Am. J. Pathol. 1939;15:89–110.7. [PMC free article] [PubMed] [Google Scholar]
- 39.Meddeb M., Chow R.D., Whipps R., Haque R. The heart as a site of metastasis of benign metastasizing leiomyoma: case report and review of the literature. Case Rep. Cardiol. 2018 May;21(2018) doi: 10.1155/2018/7231326. PMID: 29951323; PMCID: PMC5987332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller J., Shoni M., Siegert C., Lebenthal A., Godleski J., McNamee C. Benign metastasizing leiomyomas to the lungs: an institutional case series and a review of the recent literature. Ann. Thorac. Surg. 2016 Jan;101(1):253–258. doi: 10.1016/j.athoracsur.2015.05.107. [DOI] [PubMed] [Google Scholar]
- 41.Grafino M., Ferreira L., Telo L., Mira S., Alvoeiro M., Bárbara C. A rare cause of miliary pattern and respiratory failure - benign metastasizing leiomyoma. Rev. Port. Pneumol. 2016 Sep-Oct;22(5):296–297. doi: 10.1016/j.rppnen.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 42.Lam P.M., Lo K.W., Yu M.Y., Wong W.S., Lau J.Y., Arifi A.A., Cheung T.H. Intravenous leiomyomatosis: two cases with different routes of tumor extension. J. Vasc. Surg. 2004 Feb;39(2):465–469. doi: 10.1016/j.jvs.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 43.Clay T.D., Dimitriou J., McNally O.M., Russell P.A., Newcomb A.E., Wilson A.M. Intravenous leiomyomatosis with intracardiac extension - a review of diagnosis and management with an illustrative case. Surg. Oncol. 2013 Sep;22(3):e44–e52. doi: 10.1016/j.suronc.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Yu X., Zhang G., Lang J., Liu B., Zhao D. Factors associated with recurrence after surgical resection in women with intravenous leiomyomatosis. Obstet. Gynecol. 2016 Nov;128(5):1018–1024. doi: 10.1097/AOG.0000000000001718. [DOI] [PubMed] [Google Scholar]
- 45.Yu X., Fu J., Cao T., Huang L., Qie M., Ouyang Y. Clinicopathologic features and clinical outcomes of intravenous leiomyomatosis of the uterus: a case series. Medicine (Baltimore) 2021 Jan 8;100(1) doi: 10.1097/MD.0000000000024228. PMID: 33429819; PMCID: PMC7793403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang G., Yu X., Lang J. Intravenous leiomyomatosis with inferior vena cava or intracardiac extension and concurrent bilateral multiple pulmonary nodules: a report of 2 cases. Medicine (Baltimore) 2016 Aug;95(35) doi: 10.1097/MD.0000000000004722. PMID: 27583911; PMCID: PMC5008595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barnas E., Ras R., Skret-Magierlo J., Wesecki M., Filipowska J., Ksiazek M., Skret A., Widenka K. Natural history of leiomyomas beyond the uterus. Medicine (Baltimore) 2019 Jun;98(25) doi: 10.1097/MD.0000000000015877. PMID: 31232922; PMCID: PMC6636938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Low H.Y., Zhao Y., Huang K.S., Shen H.P., Wu P.J., Tseng C.J. Intravenous leiomyomatosis of the uterus: a clinicopathological analysis of nine cases and literature review. Taiwan J Obstet Gynecol. 2017 Jun;56(3):362–365. doi: 10.1016/j.tjog.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 49.Mizoguchi C., Matsumoto H., Nasu K., Arakane M., Kai K., Narahara H. Intravenous leiomyomatosis treated with radical hysterectomy and adjuvant aromatase inhibitor therapy. J. Obstet. Gynaecol. Res. 2016 Oct;42(10):1405–1408. doi: 10.1111/jog.13063. [DOI] [PubMed] [Google Scholar]
- 50.Biri A., Korucuoglu U., Zumrutbas N., Tiras B., Guner H. Intravenous leiomyomatosis treated with aromatase inhibitor therapy. Int. J. Gynaecol. Obstet. 2008 Jun;101(3):299–300. doi: 10.1016/j.ijgo.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Mitsuhashi A., Nagai Y., Sugita M., Nakajima N., Sekiya S. GnRH agonist for intravenous leiomyomatosis with cardiac extension. A case report. J. Reprod. Med. 1999 Oct;44(10):883–886. [PubMed] [Google Scholar]
- 52.Rosa P., Pidhorecky I. A case of intravenous leiomyomatosis with involvement of a renal vein. Ann. Vasc. Surg. 2018 Nov;53:271.e11–271.e13. doi: 10.1016/j.avsg.2018.05.061. [DOI] [PubMed] [Google Scholar]
- 53.Mata R.P., Urzal C., Belo A.I., Guerreiro F. Intravenous leiomyomatosis without extrapelvic involvement. BMJ Case Rep. 2020 Mar 18;13(3) doi: 10.1136/bcr-2020-234864. PMID: 32193185; PMCID: PMC7101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Awonuga A.O., Shavell V.I., Imudia A.N., Rotas M., Diamond M.P., Puscheck E.E. Pathogenesis of benign metastasizing leiomyoma: a review. Obstet Gynecol Surv. 2010 Mar;65(3):189–195. doi: 10.1097/OGX.0b013e3181d60f93. [DOI] [PubMed] [Google Scholar]
- 55.Rivera J.A., Christopoulos S., Small D., Trifiro M. Hormonal manipulation of benign metastasizing leiomyomas: report of two cases and review of the literature. J. Clin. Endocrinol. Metab. 2004 Jul;89(7):3183–3188. doi: 10.1210/jc.2003-032021. [DOI] [PubMed] [Google Scholar]
- 56.Lewis E.I., Chason R.J., AH DeCherney, Armstrong A., Elkas J., Venkatesan A.M. Novel hormone treatment of benign metastasizing leiomyoma: an analysis of five cases and literature review. Fertil. Steril. 2013 Jun;99(7):2017–2024. doi: 10.1016/j.fertnstert.2013.01.147. Epub 2013 Mar 5. PMID: 23465706; PMCID: PMC3672263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Correia P., Castro A., Rocha A., Freitas D., Carnide C., Moutinho O. Pelvic intravenous leiomyomatosis - case report. Rev. Bras. Ginecol. Obstet. 2016 Aug;38(8):412–415. doi: 10.1055/s-0036-1588002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chan M.G., Huang K.G. Intravenous leiomyomatosis in the parametrium. J. Minim. Invasive Gynecol. 2016 Sep-Oct;23(6):849–850. doi: 10.1016/j.jmig.2016.01.015. Epub 2016 Jan 21. [DOI] [PubMed] [Google Scholar]
- 59.Bayya J., Minkoff H., Khulpateea N. Tamoxifen and growth of an extrauterine leiomyoma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008 Nov;141(1):90–91. doi: 10.1016/j.ejogrb.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Y., Clark L.H., Sheng X., Zhou C. Successful en bloc venous resection with reconstruction and subsequent radiotherapy for 2 consecutive recurrences of intravenous leiomyoma–a case report. BMC Cancer. 2016 Jan;6(16):6. doi: 10.1186/s12885-015-2045-8. PMID: 26739818; PMCID: PMC4704417. <comment>PMID: 26739818; PMCID: PMC4704417</comment>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang G., Feng F., Wang W., Zhu L. Rapamycin (sirolimus) in treatment of recurrent intravenous leiomyomatosis: a case report. BJOG. 2020 May;127(6):768–771. doi: 10.1111/1471-0528.16156. [DOI] [PubMed] [Google Scholar]
- 62.Chen S., Zhang Y., Zhang J., Hu H., Cheng Y., Zhou J., Shen L., Chen H. Pulmonary benign metastasizing leiomyoma from uterine leiomyoma. World J. Surg. Oncol. 2013 Jul;18(11):163. doi: 10.1186/1477-7819-11-163. PMID: 23866077; PMCID: PMC3722006. [DOI] [PMC free article] [PubMed] [Google Scholar]