Abstract
Background
Hypogonadism associated with cancer is reported to cause cachexia and a variety of physical and psychological symptoms. This study aims to evaluate whether androgen replacement therapy can improve cancer‐related symptoms in male advanced cancer patients.
Methods
An investigator‐initiated, prospective, and randomized controlled study was conducted. Patients with low serum testosterone levels (total or free testosterone levels were <2.31 ng/mL or <11.8 pg/mL, respectively) were randomly assigned to the control or testosterone enanthate administration (testosterone group) groups. Testosterone enanthate was injected into the muscle tissue at a dose of 250 mg every 4 weeks (baseline, week 4, and week 8). Differences in quality of life questionnaires and cachexia‐related serum protein levels between groups were assessed.
Results
This study enrolled and randomized 106 and 81 patients, respectively. Moreover, 41 and 40 patients were in the control and testosterone groups, respectively. Although no significant differences in the change of subscales and total scores in Functional Assessment of Anorexia/Cachexia Treatment were noted from the baseline between the two groups, the testosterone group showed a significantly better change in the ‘unhappiness’ item of the Edmonton Symptom Assessment System at week 12 compared with baseline versus the control group (−1.4 and 0.0 points, respectively; mean, P = 0.007). No significant differences exist in the change of serum interleukin‐6 and insulin‐like growth factor‐1 levels at week 12 from the baseline between the control and testosterone groups. Consequently, the testosterone group significantly inhibited the change in serum tumour necrotic factor‐α level at week 12 from the baseline compared with the control group (+0.4 and +0.1 pg/mL, respectively; mean, P = 0.005).
Conclusions
Although testosterone enanthate did not improve most of the items in health‐related quality of life questionnaires, testosterone enanthate induced a significantly better change in the ‘unhappiness’ item at week 12 compared with the control. Testosterone enanthate may be a potential treatment option for male advanced cancer patients.
Keywords: Advanced cancer, Androgen replacement therapy, Cachexia, Health‐related quality of life, Male hypogonadism
Introduction
Health‐related quality of life during anticancer treatment is an important consideration although prolonging life is the main goal of cancer treatment. Anticancer treatments, such as a conventional cytotoxic chemotherapeutic, molecular targeting therapeutic agents, and, recently, immune checkpoint inhibitors, contribute to survival time improvement in advanced cancer patients with non‐curative locally advanced or metastatic lesions. However, many symptoms are induced by cancer itself or as the adverse events of anticancer agents' burden patients regardless of the cancer type. Various supportive care measures are needed to relieve such symptoms during the limited life period. Recent studies revealed that low serum testosterone level is associated with advanced cancer and induces many cancer‐related symptoms (e.g. depression, weight loss, albumin loss, sarcopenia, worsening of pain, and increase of opioid dose). Moreover, these symptoms overlap with those of cachexia. 1 , 2 , 3 , 4 , 5 Consequently, these symptoms cause a severe deterioration in health‐related quality of life. Thus, intensive cancer supportive care is needed. Each symptom is currently individually treated with a specific therapy. However, hypogonadism may be theoretically treated by androgen replacement therapy, and most symptoms are resolved at once. Some studies demonstrated that palliative care enhances mood, patient satisfaction, quality of end‐of‐life care, and even survival. 6 , 7 , 8 , 9 However, whether androgen replacement therapy can improve cancer‐related symptoms including cachexia and health‐related quality of life is yet unclear. The ARTFORM study planned to prospectively examine the efficacy of androgen replacement therapy by testosterone enanthate injection on cancer‐related symptoms in male advanced cancer patients. 10
Materials and methods
Study design and conduct
The ARTFORM study is an investigator‐initiated, randomized controlled trial evaluating the efficacy of testosterone enanthate in male advanced cancer patients with non‐curative locally advanced or metastatic lesions. The efficacy of testosterone enanthate was assessed using validated health‐related quality of life questionnaires and serum cachexia‐related biomarkers for 12 weeks. The ARTFORM study received approval from the Institutional Review Board of Kanazawa University. Consequently, the trial was registered in the University Hospital Medical Information Network (centre identifier UMIN 000010939). The study was conducted following the 1975 Declaration of Helsinki (revised, 2013). All the patients provided written informed consent before registration.
Patient recruitment and randomization
Male patients with advanced cancer who consulted the Department of Urology and Division of Medical Oncology, Kanazawa University Hospital, were enrolled between 15 June 2013 and 31 December 2017. Total (<2.31 ng/mL) and free (<11.8 pg/mL) testosterone levels with the presence of symptoms were reported as a standard diagnostic value for late‐onset hypogonadism in men internationally and Japan, respectively. 11 , 12 Patients had their serum total and free testosterone levels checked. Those with total and free testosterone levels ≥2.31 ng/mL and 11.8 pg/mL, respectively, were excluded from randomization. Patients were randomly assigned to either the non‐administration (control group) or testosterone enanthate administration (testosterone group) groups if either total or free testosterone level was <2.31 ng/mL or <11.8 pg/mL, respectively. A block randomization method was applied to equally assign patient numbers to each group. 13 A technical staff who is not an investigator of the ARTFORM study determined the block size (two, four, or six including equal numbers) of each group and then places the blocks so that the investigators cannot predict the next assignment.
Eligibility criteria
Inclusion criteria required patients to be male, have a pathologically or cytologically confirmed cancer, have non‐curative locally advanced or metastatic lesions, know their diagnosis, provide written informed consent, and have appropriate hepatic and renal functionality as demonstrated in laboratory tests within 4 weeks before registration [aspartate transaminase ≤3.0 × upper limit of normal (ULN), alanine transaminase ≤3.0 × ULN, blood urea nitrogen ≤30 mg/dL, and serum creatinine ≤3.0 mg/dL]. Patients were excluded if they were <20 years old; had prostate cancer, previous history of the disease, or severe benign prostate hyperplasia defined as prostate volume >40 mL with a history of acute urinary retention; had possible prostate cancer with serum prostate‐specific antigen (PSA) level >4.0 ng/mL; or were considered by a principal or clinical investigator to be inappropriate for participation in the present study for any other reason.
Data collection
The patients were asked to provide a complete medical history after providing written informed consent to participate in the ARTFORM study. Clinical data obtained in the study included the Eastern Cooperative Oncology Group Performance Status (PS), physical measurements (e.g. age, height, and body weight), primary cancer status and treatment, haematological measurements [e.g. white blood cell, red blood cell, haemoglobin (Hb), and platelet counts], general blood biochemical measurements (e.g. creatinine, liver enzymes, and electrolytes), hormonal measurements [total and free testosterone, PSA, luteinizing hormone (LH), and follicle‐stimulating hormone (FSH)], and representative cachexia‐related biomarkers [i.e. interleukin‐6 (IL‐6), tumour necrotic factor‐α (TNF‐α), and insulin‐like growth factor‐1 (IGF‐1)]. Examinations were performed every 4 weeks for 12 weeks. PSA was measured at weeks 0 (baseline) and 12, and cachexia‐related biomarkers were measured at baseline, week 4, and week 12. However, these examinations could be performed at any time if a principal or clinical investigator considered these examinations to be necessary. Blood samples in all patients were collected before 10 am. Moreover, total testosterone was measured by electrochemiluminescence immunoassay. Similarly, free testosterone was measured by solid‐phase radioimmunoassay between 15 June 2013 and 31 January 2015 and then by enzyme immunoassay between 1 February and 31 December 2017. These two methods were confirmed to be equivalent by BML (Tokyo, Japan). IL‐6, TNF‐α, and IGF‐1 were measured by chemiluminescent enzyme immunoassay, enzyme‐linked immunosorbent assay, and immune radiometric assay, respectively. Validated health‐related quality of life questionnaires, the Edmonton Symptom Assessment System (ESAS), and the Functional Assessment of Anorexia/Cachexia Treatment (FAACT) version 4, translated into Japanese, were administered at baseline and weeks 4, 8, and 12 after treatment commenced to comprehensively evaluate the various aspects of physical and psychosocial well‐being. 14 , 15 The primary endpoint was the difference in change of health‐related quality of life at week 12 compared with baseline as measured by these questionnaires between the control and testosterone groups. The difference in change of cachexia‐related biomarkers was set as an important exploratory endpoint.
Assessment of Edmonton Symptom Assessment System and Functional Assessment of Anorexia/Cachexia Treatment
Edmonton Symptom Assessment System is a simple method to assess the health‐related quality of life consisting of 10 items. The patients can intuitively score each item on a scale of 0–10. A higher ESAS score indicates a worse health‐related quality of life. 14 Moreover, FAACT is designed to measure the general aspects of quality of life as well as specific anorexia/cachexia‐related concerns in detail and consists of five subscales including 39 items: physical well‐being (PWB), social well‐being (SWB), emotional well‐being (EWB), functional well‐being (FWB), and anorexia cachexia subscale (ACS). FAACT trial outcome score, Functional Assessment of Cancer Therapy‐General total score, and FAACT total score were calculated as PWB + FWB + ACS, PWB + SWB + EWB + FWB and PWB + SWB + EWB + FWB + ACS, respectively. Contrary to ESAS, higher FAACT scores indicate a better health‐related quality of life. 15
Administration of testosterone enanthate
Testosterone enanthate was injected into the muscle tissue of patients who are assigned to the testosterone group at a dose of 250 mg every 4 weeks (baseline, week 4, and week 8). This treatment schedule was designed taking accessibility and convenience for outpatients and health insurance coverage in Japan into consideration. The administration of testosterone enanthate was scheduled to terminate when any of the following events occurred: (i) the patient withdrew written informed consent or opted out of the study; (ii) the patient was no longer eligible for the study; (iii) cancer was completely cured; (iv) the patient's condition deteriorated and testosterone enanthate was not appropriate; (v) severe adverse events occurred; (vi) the patient's compliance was poor; or (vii) the patient's participation was considered inappropriate by a principal or clinical investigator for any other reason. Testosterone enanthate‐related adverse events were particularly collected because patients in the control group did not undergo a placebo treatment and were observed during the study period. Any treatments for primary cancer including chemotherapy, immunotherapy, surgery, and radiotherapy as well as supportive care measures were allowed during the ARTFORM study.
Assessment of overall survival
Although palliative and supportive care in cancer are usually offered not to eradicate cancer itself but to improve quality of life and symptoms, some studies demonstrated that palliative care enhances mood, patient satisfaction, quality of end‐of‐life care, and even survival. 6 , 7 , 8 , 9 However, most studies did not show the survival benefit of palliative and supportive care, and whether overall survival can be prolonged by such care is still controversial. Therefore, overall survival was investigated as an exploratory endpoint in the current study. Moreover, survival was calculated from the date patients were randomized to the date of death from any cause. Any treatments for primary cancer including chemotherapy, immunotherapy, surgery, and radiotherapy as well as supportive care measures including testosterone enanthate were also allowed after 12 weeks of the study.
Statistical analyses
Statistical analyses were performed using commercially available software GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA). The Shapiro–Wilk test was used to assess if the values come from a Gaussian distribution. Unpaired t‐tests or Mann–Whitney tests were used to analyse the difference of scores in health‐related quality of life questionnaires and serum cachexia‐related biomarkers between the two groups. Paired t‐tests or Wilcoxon signed‐rank tests were used to analyse differences of scores in health‐related quality of life questionnaires between baseline and each time; chronological changes; haematological and blood biochemical measurements; and serum cachexia‐related biomarkers. Fisher's exact test, χ 2 test, and χ 2 test for trend were used to analyse contingency tables. Overall survival was estimated using the Kaplan–Meier method. The log‐rank test was used to compare survival distributions. Statistical significance was defined as P < 0.05.
Results
Patients
The ARTFORM study enrolled 106 male advanced cancer patients. Before randomization, 25 patients were excluded due to normal total and free testosterone levels (13), high PSA levels (7), renal dysfunction (4), and consent withdrawal (1). Of 81 randomized patients, 41 and 40 were assigned to the control and testosterone groups, respectively. During 12 weeks of the study, one and three patients in the control and testosterone groups expired. Moreover, one patient terminated treatment in the testosterone group because of liver injury. In addition, another patient made the decision to terminate treatment. Finally, 40 and 35 control and testosterone patients, respectively, reached a 12‐week analysis. The trial profile is shown in Figure 1.
Figure 1.
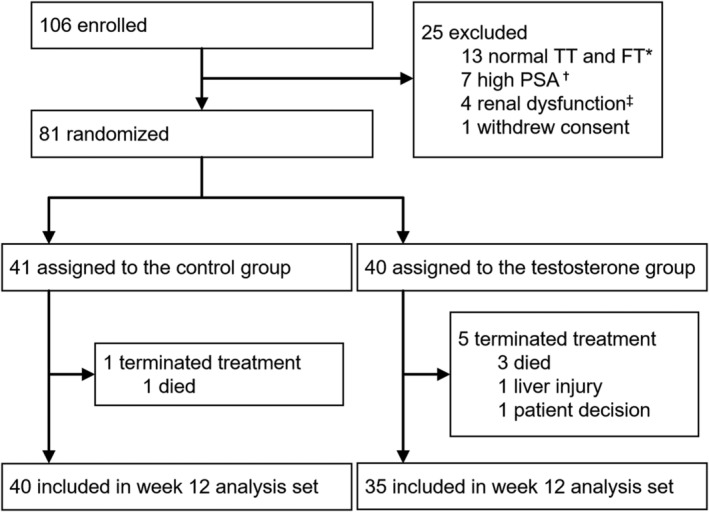
Trial profile. The ARTFORM study enrolled 106 male advanced cancer patients. Before randomization, 25 patients were excluded. Of 81 randomized patients, 41 and 40 were assigned to the control and testosterone groups, respectively. Testosterone enanthate was injected into the muscle tissue of patients who are assigned to the testosterone group at a dose of 250 mg every 4 weeks (baseline, weeks 4, and weeks 8). Finally, 40 and 35 control and testosterone patients, respectively, reached a 12‐week analysis. TT = total testosterone; FT = free testosterone; PSA = prostate‐specific antigen. *Patients with total and free testosterone levels ≥2.31 ng/mL and 11.8 pg/mL, respectively, were excluded from randomization. †Patients with PSA level >4.0 ng/mL were excluded from randomization. ‡Patients with blood urea nitrogen >30 mg/dL, and serum creatinine >3.0 mg/dL were excluded from randomization.
The background of the patients is shown in Table 1. PS was significantly higher in the testosterone group compared with the control group (P = 0.006). Previous body weight data (6–12 months before registration) were available in 15 and 12 patients in the control and testosterone groups, respectively. Bodyweight at baseline and between 6 and 12 months before registration was compared although data were not available for the majority of the subjects. Patients in the testosterone group lost more weight compared with the control group (P = 0.032). However, no significant difference in body mass index at baseline exists between the control and testosterone groups. The number of patients who had free testosterone levels <11.8 pg/mL in the control group and the testosterone group was 37 and 40, respectively. Moreover, no significant differences were noted in other baseline values including cachexia‐related serum proteins between the two groups. Genitourinary cancer included kidney, renal pelvic, ureteral, and bladder cancer, and other primary malignancies included lung, pancreatic, gallbladder, bile duct, thyroid, and unknown primary cancers as well as malignant mesothelioma, leukaemia, and retroperitoneal sarcoma.
Table 1.
Patient background
| Control group | Testosterone group | P‐value | |||
|---|---|---|---|---|---|
| n | 41 | 40 | |||
| Age, year | 68 (49–94) | 67 (52–83) | n.s. | ||
| BMI | 22.1 (17.6–32.6) | 21.4 (15.3–30.6) | n.s. | ||
| PS, n | 0 | 25 | 19 | 0.006 | |
| 1 | 15 | 9 | |||
| 2 | 1 | 7 | |||
| 3 | 0 | 4 | |||
| 4 | 0 | 1 | |||
| Primary site, n | GU | Kidney | 14 | 16 | n.s. |
| Bladder | 6 | 4 | |||
| Renal pelvis | 3 | 3 | |||
| Ureter | 2 | 3 | |||
| Others | Pancreas | 7 | 3 | ||
| Lung | 5 | 5 | |||
| STS | 2 | 0 | |||
| MM | 1 | 1 | |||
| Gallbladder | 0 | 1 | |||
| Bile duct | 0 | 1 | |||
| Thyroid | 0 | 1 | |||
| Leukaemia | 0 | 1 | |||
| CUP | 1 | 1 | |||
| Disease status, n | Locally invasive | 4 | 2 | n.s. | |
| Metastatic | 36 | 36 | |||
| Undefined | 1 | 2 | |||
| Concurrent cancer treatment, n | Chemotherapy | 22 | 18 | n.s. | |
| Molecular targeted therapy | 14 | 16 | |||
| Radiotherapy | 3 | 3 | |||
| None | 2 | 3 | |||
| Surgery for primary site, n | Yes | 21 | 17 | n.s. | |
| No | 20 | 23 | |||
| Previous treatment line a , n | 0 | 13 | 15 | n.s. | |
| 1 | 9 | 8 | |||
| 2 | 11 | 9 | |||
| 3 | 6 | 5 | |||
| 4 | 2 | 3 | |||
| Antidepressive/antianxiety agent, n | Yes | 10 | 11 | n.s. | |
| No | 31 | 29 | |||
| Past weight data b , n | Not available | 26 | 28 | ||
| Available | 15 | 12 | |||
| Weight change, kg | −0.7 (−6.2 − +6.4) | −2.65 (−23.6 − +6.3) | 0.032 | ||
| TT, ng/mL | 4.0 (0.3–8.4) | 3.7 (0.2–12.2) | n.s. | ||
| FT, pg/mL | 5.6 (3.6–15.8) | 5.2 (0.2–11.6) | n.s. | ||
| PSA, ng/mL | 1.1 (0.0–4.0) | 0.9 (0.0–3.3) | n.s. | ||
| LH, mIU/mL | 12.1 (7.6–127) | 9.8 (4.1–57.6) | n.s. | ||
| FSH, mIU/mL | 21.8 (11.6–182) | 19.3 (5.4–91.7) | n.s. | ||
| Hb, g/dL | 10.9 (6.8–16.6) | 11.7 (7.5–15.1) | n.s. | ||
| WBC, ×103/μL | 5.5 (1.5–57.8) | 6.0 (1.8–10.1) | n.s. | ||
| Plt, ×104/μL | 20.5 (4.8–90.6) | 23.4 (9.5–80.2) | n.s. | ||
| CRP, mg/dL | 0.9 (0.0–13.0) | 0.9 (0.0–18.8) | n.s. | ||
| Cr, mg/mL | 0.9 (0.2–2.7) | 0.8 (0.5–18.8) | n.s. | ||
| Ca, mg/dL | 9.1 (8.2–10.7) | 9.1 (7.9–11.0) | n.s. | ||
| LDH, U/L | 188 (114–494) | 184 (118–756) | n.s. | ||
| TP, g/dL | 6.6 (5.0–7.7) | 6.6 (4.2–8.1) | n.s. | ||
| Alb, g/dL | 3.6 (2.0–4.7) | 3.6 (2.3–4.4) | n.s. | ||
| IL‐6, pg/mL | 8.0 (0.6–202) | 7.3 (0.8–107) | n.s. | ||
| TNF‐α, pg/mL | 1.6 (0.5–5.8) | 1.9 (0.5–17.9) | n.s. | ||
| IGF‐1, ng/mL | 119 (39–251) | 102 (38–239) | n.s. | ||
Median values are shown except for indicated as n. Values in parentheses indicate range.
Alb, albumin; BMI, body mass index; Ca, calcium; Cr, creatinine; CRP, C‐reactive protein; CUP, carcinoma of unknown primary; FSH, follicle‐stimulating hormone; FT, free testosterone; GU, genitourinary; Hb, haemoglobin; IGF‐1, insulin‐like growth factor‐1; IL‐6, interleukin‐6; LDH, lactate dehydrogenase; LH, luteinizing hormone; MM, malignant mesothelioma; n.s., not significant; Plt, platelet; PS, performance status; PSA, prostate‐specific antigen; STS, soft tissue sarcoma; TNF‐α, tumour necrotic factor‐α; TP, total protein; TT, total testosterone; WBC, white blood cell.
Cancer treatment includes any anticancer treatments for primary cancer such as chemotherapy, molecular targeted therapy, surgery, and radiotherapy.
6–12 months before registration.
Health‐related quality of life questionnaires
The numbers of patients who answered health‐related quality of life questionnaires in the control and testosterone groups at baseline and weeks 4, 8, and 12 were 41 and 40, 41 and 40, 39 and 37, and 39 and 35, respectively. One patient in the control group did not answer the health‐related quality of life questionnaires at weeks 8 and 12.
Table 2 shows the change of average ESAS score and FAACT subscale and total scores from baseline. However, no significant differences in the FAACT subscale and total scores were noted. Moreover, the testosterone group showed a significantly better change in the ‘unhappiness’ item of the ESAS at week 12 compared with baseline versus the control group (−1.4 and 0.0, respectively; mean, P = 0.007). Average ESAS scores and FAACT subscale and total scores at each time point are shown in Supporting Information, Table S1.
Table 2.
The change of average ESAS score, and FAACT subscale and total scores from baseline
| At week 4 | At week 8 | At week 12 | P‐value (control vs. testosterone at week 12) | ||||
|---|---|---|---|---|---|---|---|
| Control group | Testosterone group | Control group | Testosterone group | Control group | Testosterone group | ||
| ESAS item a | |||||||
| Pain | −0.5 | −0.5 | −0.1 | −0.4 | −0.1 | −0.5 | n.s. |
| Fatigue | 0.7 | −0.1 | 0.3 | 0.3 | 0.5 | 0.2 | n.s. |
| Nausea | 0.0 | 0.3 | 0.6 | 0.0 | 0.4 | −0.1 | n.s. |
| Depression | 0.4 | −0.1 | 0.4 | 0.1 | 0.6 | 0.2 | n.s. |
| Anxiety | −0.2 | 0.9 | −0.1 | 0.3 | −0.3 | 0.0 | n.s. |
| Drowsiness | 0.0 | 0.4 | −0.2 | 0.1 | −0.7 | 0.0 | n.s. |
| Appetite loss | −0.1 | −0.3 | 0.1 | −0.4 | −0.7 | −0.4 | n.s. |
| Unhappiness | 0.1 | −0.5 | 0.0 | −0.9 | 0.0 | −1.4 | 0.007 |
| Dyspnoea | 0.2 | 0.5 | 0.2 | 0.5 | 0.2 | 0.3 | n.s. |
| Other problem | 0.6 | 0.6 | 0.4 | 0.4 | 0.4 | 0.3 | n.s. |
| Total | 0.5 | 0.1 | 2.4 | −0.8 | 0.3 | −1.6 | n.s. |
| FAACT subscale/total b | |||||||
| PWB | 0.0 | −0.7 | −0.1 | −0.3 | −0.5 | 0.1 | n.s. |
| SWB | −2.1 | −0.8 | −2.4 | −1.2 | −2.0 | −0.9 | n.s. |
| EWB | 0.4 | −0.8 | 1.3 | −0.5 | 0.5 | −0.8 | n.s. |
| FWB | −0.6 | −1.5 | −3.0 | −2.0 | −2.0 | −1.2 | n.s. |
| ACS | −1.1 | 0.1 | −0.4 | 0.8 | −0.2 | −0.3 | n.s. |
| FAACT TOI | −1.7 | −2.1 | −3.5 | −1.5 | −2.7 | −1.4 | n.s. |
| FACT‐G Total | −2.2 | −3.8 | −4.2 | −4.0 | −4.1 | −2.8 | n.s. |
| FAACT Total | −3.3 | −3.7 | −4.6 | −3.2 | −4.3 | −3.1 | n.s. |
ACS, anorexia cachexia subscale; EWB, emotional well‐being; FACT‐G, Functional Assessment of Cancer Therapy‐General; FWB, functional well‐being; n.s., not significant; PWB, physical well‐being; SWB, social/family well‐being; TOI, trial outcome index.
The higher the score, the worse the health‐related quality of life.
he higher the score, the better the health‐related quality of life.
Testosterone‐related measurements in the blood
The change of testosterone‐related measurements in the blood is shown in Figure 2. Total testosterone levels in both control and testosterone groups did not significantly change through the study period. Meanwhile, free testosterone levels in both control and testosterone groups significantly decreased at week 8 [from 6.4 ± 0.6 pg/mL at baseline to 5.5 ± 0.6 pg/mL at week 8 in the control group, P = 0.037; 5.6 ± 0.4 pg/mL at baseline to 4.4 ± 0.4 pg/mL at week 8 in the testosterone group, P = 0.048; mean ± standard error of the mean (SEM), the same shall apply hereinafter]. The activation of the androgen‐targeting cells reflects the Hb levels in the control group showing a significant decrease at weeks 4 and 8 (from 11.3 ± 0.3 pg/mL at baseline to 10.6 ± 0.3 pg/mL at week 4 and 10.4 ± 0.3 pg/mL at week 8; P = 0.003 and 0.003, respectively). However, PSA levels, reflecting the activation of the androgen‐targeting cells, did not significantly change through the study period. Moreover, both LH and FSH levels in the testosterone group were significantly decreased at all points (from 14.8 ± 1.9 mIU/mL at baseline to 9.2 ± 1.3, 8.8 ± 1.4, and 10.5 ± 1.8 mIU/mL at weeks 4, 8, and 12, respectively, for LH (P = 0.002, <0.001, and 0.007, respectively), and from 26.1 ± 3.0 mIU/mL at baseline to 18.3 ± 2.2, 17.4 ± 2.9, and 21.4 ± 3.3 mIU/mL at weeks 4, 8, and 12 for FSH (P = 0.017, 0.002, and 0.043, respectively), clearly suggesting that testosterone enanthate acted on the patient's body and induced negative feedback of LH and FSH. Conversely, FSH levels in the control group increased at weeks 8 and 12 (from 29.9 ± 4.8 mIU/mL at baseline to 30.7 ± 3.3 and 35.9 ± 5.2 mIU/mL at weeks 8 and 12, respectively; P < 0.001 and <0.001, respectively).
Figure 2.
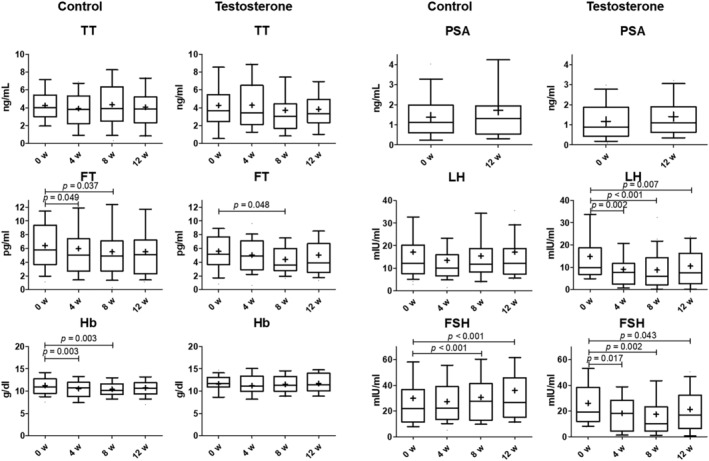
The change of testosterone‐related measurements in blood in the control and testosterone group. The number of patients included in the control and testosterone groups at baseline, weeks 4, 8, and 12 were 41 and 40, 41 and 40, 40 and 37, and 40 and 35, respectively. Data were analysed using paired t‐tests or Wilcoxon signed‐rank tests after the Shapiro–Wilk test. Boxes indicate between lower and upper quartiles, whiskers indicate 10–90 percentile, and horizontal bars and ‘+’ in boxes indicate median and mean, respectively. Statistical significance was defined as P < 0.05. TT = total testosterone; FT = free testosterone; Hb = haemoglobin; PSA = prostate‐specific antigen; LH = luteinizing hormone; FSH = follicle‐stimulating hormone.
Cachexia‐related serum proteins
The changes of cachexia‐related serum proteins are shown in Figure 3. The IL‐6 levels in both control and testosterone groups did not significantly change in the study period (Figure 3). In the control group, serum TNF‐α level was significantly increased at week 12 (from 1.8 ± 0.2 pg/mL at baseline to 2.1 ± 0.2 pg/mL at week 12; P = 0.002). However, the increase of serum TNF‐α level was not observed in the testosterone group (Figure 3). Generally, the higher serum TNF‐α level indicates a greater cachexic status. 16 , 17 Similarly, although serum IGF‐1 level in the control group was significantly decreased at week 12 (from 120.1 ± 7.3 ng/mL at baseline to 101.6 ± 6.8 ng/mL at week 12; P = 0.003), the decrease of serum IGF‐1 level was not observed in the testosterone group (Figure 3). Generally, lower serum IGF‐1 levels indicate a greater cachexic status. 18 The testosterone group significantly inhibited the change in serum TNF‐α level at week 12 compared with the control group although no significant differences exist in the change of IL‐6 and IGF‐1 levels at week 12 from baseline between the control and testosterone groups (+0.4 and +0.1 pg/mL, respectively; mean, P = 0.005; Table 3).
Figure 3.
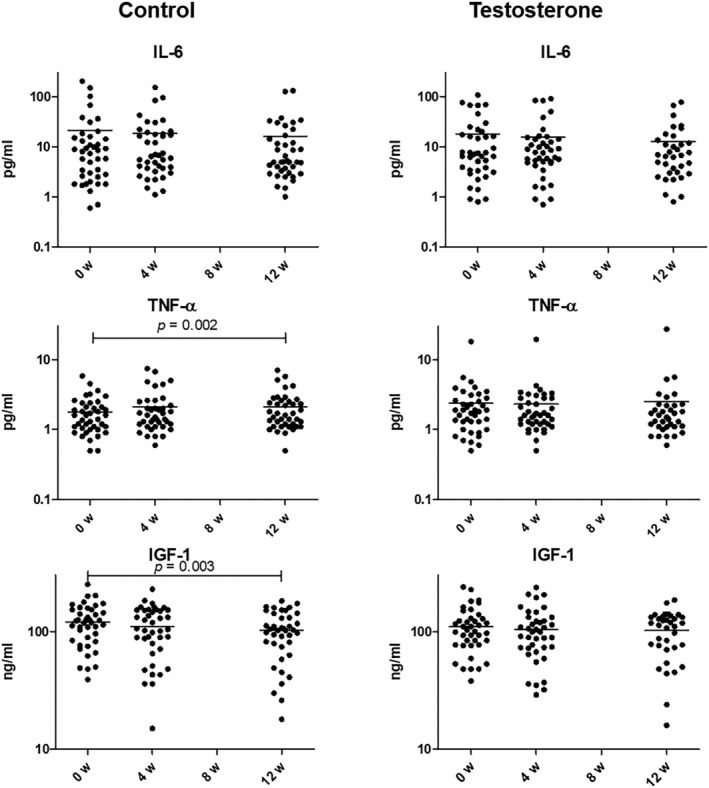
The changes of cachexia‐related serum proteins in the control and testosterone groups. Chronological changes are shown in semi‐log plot. The number of patients included in the control and testosterone groups at baseline, weeks 4, and 12 were 41 and 40, 41 and 40, and 40 and 35, respectively. Data were analysed using paired t‐tests or Wilcoxon signed‐rank tests after the Shapiro–Wilk test. Horizontal bars indicate mean, values. IL‐6 = interleukin‐6; TNF‐α = tumour necrotic factor‐α; IGF‐1 = insulin‐like growth factor‐1.
Table 3.
The change of average serum IL‐6, TNF‐α, and IGF‐1 from baseline
| At week 4 | At week 12 | P‐value (control vs. testosterone at week 12) | |||
|---|---|---|---|---|---|
| Control group | Testosterone group | Control group | Testosterone group | ||
| IL‐6, pg/mL | −3.1 | −2.7 | −2.8 | −4.7 | n.s. |
| TNF‐α, pg/mL | 0.4 | −0.1 | 0.4 | 0.1 | 0.005 |
| IGF‐1, ng/mL | −10.0 | −5.4 | −18.7 | −12.4 | n.s. |
n.s., not significant; IL‐6, interleukin‐6; TNF‐α, tumour necrotic factor‐α; IGF‐1, insulin‐like growth factor‐1.
Treatment‐related adverse events and overall survival
Testosterone enanthate‐related adverse events were collected in the testosterone group during the study period. Grade 3 (Common Terminology Criteria for Adverse Events v4.0) aspartate transaminase and alanine transaminase increases were observed in one patient. During the study period, one and three mortalities caused by cancer progression were observed in the control and testosterone groups, respectively. The Kaplan–Meier curves of overall survival in both groups are shown in Figure 4. The survival rates of 63.1% and 61.0% at 12 and 42.4% and 42.5% at 24 months were noted in the control and testosterone groups. Moreover, no significant difference in the overall survival between the two groups was noted (P = 0.612).
Figure 4.
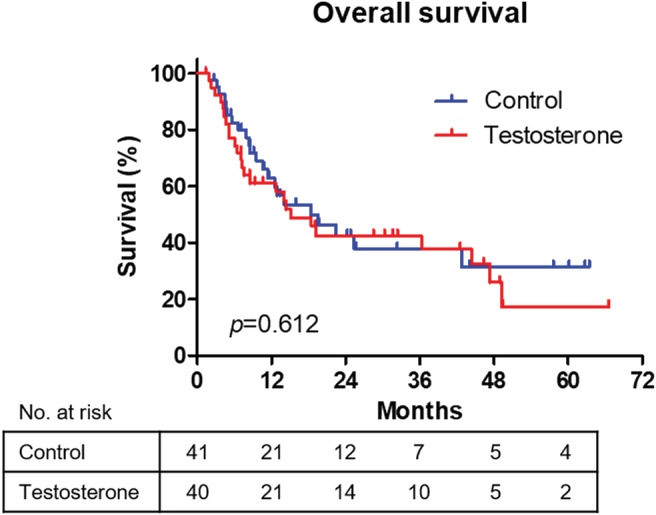
Overall survival. The Kaplan–Meier curves of overall survival in both groups are shown. The log‐rank test was used to compare survival distributions.
Discussion
The ARTFORM study showed that testosterone enanthate did not improve most of the items in health‐related quality of life questionnaires but only improved the ‘unhappiness’ item of the ESAS with minimal severe adverse events and inhibited the deterioration of serum TNF‐α levels in male advanced cancer patients. About 70% of male advanced cancer patients reportedly have hypogonadism defined as low serum testosterone levels with signs and symptoms, and low serum testosterone level could induce many cancer‐related symptoms, including cachexia. 1 , 2 , 19 Testosterone physiologically functions to maintain health in men, but the levels decrease gradually with age. 20 , 21 Testosterone improves psychological function and prevents cognitive impairment, increases bone mineral density and muscle mass, and maintains cardiovascular health. 22 , 23 , 24 , 25 , 26 However, the effect of testosterone on cognitive function and cardiovascular health is controversial because testosterone actions on vasculature are complex and have opposite effects on cerebrovascular inflammation. 27 , 28 Testosterone replacement therapy in men with cardiovascular disease should be restricted to those with the stable disease based on low‐quality evidence only after a discussion of the potential risks and benefits. 29 In addition, androgen receptors in prostate cancer cells are important drivers of cancer progression. Consequently, testosterone is associated with carcinogenesis of prostate cancer and progression of benign prostate hyperplasia. 30 , 31 , 32
Although androgen replacement therapy reportedly improves symptoms caused by hypogonadism in men, few studies show the efficacy of androgen replacement therapy on symptoms in male advanced cancer patients. 33 Thus, the ARTFORM study is the first to prospectively assess the efficacy of androgen replacement therapy from a patient perspective of direct clinical benefit using health‐related quality of life questionnaires with measurement of cachexia‐related serum protein levels. Moreover, ESAS data showed a significantly better improvement in ‘unhappiness’ at week 12 compared with baseline in the testosterone versus the control group. Furthermore, testosterone is a neuroactive steroid hormone influencing mood and appetitive behaviour. 34 A meta‐analysis showed that androgen replacement therapy is effective and efficacious in reducing depressive symptoms in men. 35 In rodent models, testosterone induces serotonin release and establishes new neuronal connections in the brain regarded as central mechanisms of action against depression. 36 , 37 , 38 , 39 Consequently, all types of supportive care (e.g. analgesics, antiemetics, and hypnotics) were allowed during the study period. These measures may have contributed to the mitigation of most physical symptoms in both groups. In addition, high PS at baseline in the testosterone group implied that cancer prognosis may be worse and cachexia incidence may be higher for this group compared with the control group. 40 , 41 However, among 11 ESAS items and eight FAACT subscales and total scores, the testosterone group showed improvement of a single ESAS item (i.e. ‘unhappiness’) at week 12 compared with the control group. Among measured cachexia‐related proteins, IL‐6 may be most important because it affects a variety of organs and cells (e.g. adipose cells and muscles). Testosterone enanthate could not decrease IL‐6 and may be the reason for the non‐improvement in most items in the health‐related quality of life questionnaires other than unhappiness. 42 In contrast, the inhibition of the increase in serum TNF‐α level and the decrease in serum IGF‐1 level in the testosterone enanthate group may contribute to the improvement of unhappiness. TNF‐α cause depressive effects in a mouse model, and TNF‐α level reduction in the hippocampus induces antidepressant‐like effects. 43 , 44 IGF‐1 alleviates depression through the CREB/PGC‐1α signal pathway activation in mice, and moreover, a high serum IGF‐1 concentration during pregnancy may help protect against postpartum depression development. 45 , 46
The nonorthodox testosterone enanthate treatment schedule in the current study may affect outcomes in terms of dose and frequency. Although several routes of delivery have been used in testosterone treatment over the years, a standard treatment regimen is not confirmed as an ideal treatment. 47 A dose range of testosterone enanthate (100–300 mg) every 2 or 3 weeks is recommended. 29 The treatment schedule applied in the current study may be insufficient to achieve a certain response in health‐related quality of life questionnaires. However, whether health‐related quality of life questionnaires could drastically improve even if a recommended dose of testosterone enanthate was administered is still unclear.
The effects of androgen replacement were confirmed in the assessment at week 4 after injection of testosterone enanthate although serum testosterone concentration rapidly decreases within 2 weeks of injection with testosterone enanthate. 48 These phenomena were evident in the change of testosterone‐related measurements in the blood. LH and FSH in the testosterone group significantly decreased despite having no change in total testosterone concentration. Moreover, serum TNF‐α level was increased at week 12 in the control group. These results support the natural progression of cancer with cachexia in advanced cancer patients recruited in the current study. Alternatively, in the testosterone group, serum TNF‐α level was not changed through the study period, suggesting that the progression of cachexia may be blocked by androgen replacement in the testosterone group although cachexia was continuously progressing in the control group. These data may support the biochemical effect of testosterone on the mechanisms of cancer cachexia in male advanced cancer patients and suggest the treatment strategy for low testosterone status, leading to potential therapeutic options for cancer cachexia. 49 However, whether the change of TNF‐α by androgen replacement therapy could affect cancer cells despite overall survival remains unclear and was not significantly different between the control and testosterone groups.
Androgen replacement therapy is well‐established with low toxicity and economic efficiency for male hypogonadism patients. 50 , 51 , 52 In the current study, grade 3 liver injury was observed in only one patient in the testosterone group. Testosterone also has some adverse effects, such as the risk of developing erythrocytosis and prostate cancer. 53 , 54 However, short‐term androgen replacement therapy seems not to affect overall survival. Injection of testosterone enanthate at a dose of 250 mg every 4 weeks is thought to be minimally invasive as a supportive care strategy for advanced cancer patients. Thus, testosterone enanthate may be a promising treatment for such patients with improvement of serum cachexia‐related proteins.
Despite several strengths, the current study had several limitations. The sample size may be insufficient to determine precise statistical significance. All patients were of Japanese ethnicity and men, and the distribution of cancers may differ in female patients or patients from other ethnic backgrounds. The scheduled administration of testosterone enanthate did not increase testosterone levels, suggesting it was subtherapeutic. The Endocrine Society and the American Urological Association recommend using a testosterone level with repeated measurements although single measurement of serum total and free testosterone levels were used for diagnosis of hypogonadism in the current study. 55 , 56 In addition, both total and free testosterone levels were not measured by the gold standard method, liquid chromatography–tandem mass spectrometry. This non‐placebo‐controlled, open‐label study has potentially biased risks in answering health‐related quality of life questionnaires as a primary endpoint. Patients in the current study were oncologically heterogeneous (e.g. >13 types of primary cancers treated with different numbers of cancer treatment and different times since diagnosis), and patient distribution between the control and testosterone groups was also uneven concerning least PS and previous weight loss. The short duration of the study period may have distorted the outcomes because overall survival was unexpectedly long in the current study. In addition, the absence of data in symptom burden, weight history, body composition, muscle mass, and function (e.g. grip strength and walking speed), some serum proteins related to cachexia, and other cachexia‐related criteria may decrease the evidential power. In particular, serum ghrelin level, reported to improve appetite, nutrition status, and skeletal muscle function, may have affected symptoms or quality of life scores. 57 , 58 Anamorelin, a novel and selective ghrelin receptor agonist, improved anorexia and nutritional status, resulting in rapid increases in body weight but failed to improve psychological parameters in patients with advanced gastrointestinal cancer who had cancer cachexia. 57 Meanwhile, the combination of ghrelin receptor agonists and androgen replacement may work complementarily to improve symptoms of cancer cachexia because androgen replacement improved psychological parameters in the current study. A large‐sized randomized controlled trial is needed as a next step to confirm the results of this study, and clinically, androgen replacement therapy is applied to male advanced cancer patients.
Conclusions
Advanced cancer patients often suffer from progressing cachexia with various physical and psychological symptoms. Moreover, testosterone enanthate did not improve most of the items in health‐related quality of life questionnaires but significantly induced a better change in the ‘unhappiness’ item of the health‐related quality of life at week 12 compared with the control, associated with the inhibition of the deterioration of serum TNF‐α levels in male advanced cancer patients.
Author contributions
Kouji Izumi had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design are caried out by Kouji Izumi and Atsushi Mizokami. All authors did the acquisition, analysis, or interpretation of data. Drafting of the manuscript is carried out by Kouji Izumi. Critical revision of the manuscript for important intellectual content is carried out by Hiroaki Iwamoto, Hiroshi Yaegashi, Takahiro Nohara, Kazuyoshi Shigehara, and Yoshifumi Kadono. Kouji Izumi did the statistical analysis. Seiji Yano and Atsushi Mizokami supervised the study.
Conflict of interest
The authors have declared no conflicts of interest.
Funding
None declared.
Supporting information
Table S1. Average ESAS scores, and FAACT subscale and total scores at each time point.
Acknowledgements
We thank Maki Morita for data collection. The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle. 59
Izumi K., Iwamoto H., Yaegashi H., Nohara T., Shigehara K., Kadono Y., Nanjo S., Yamada T., Ohtsubo K., Yano S., and Mizokami A. (2021) Androgen replacement therapy for cancer‐related symptoms in male: result of prospective randomized trial (ARTFORM study), Journal of Cachexia, Sarcopenia and Muscle, 12, 831–842, 10.1002/jcsm.12716
References
- 1. Del Fabbro E, Hui D, Dalal S, Dev R, Nooruddin ZI, Bruera E. Clinical outcomes and contributors to weight loss in a cancer cachexia clinic. J Palliat Med 2011;14:1004–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Strasser F, Palmer JL, Schover LR, Yusuf SW, Pisters K, Vassilopoulou‐Sellin R, et al. The impact of hypogonadism and autonomic dysfunction on fatigue, emotional function, and sexual desire in male patients with advanced cancer: a pilot study. Cancer 2006;107:2949–2957. [DOI] [PubMed] [Google Scholar]
- 3. Chlebowski RT, Heber D. Hypogonadism in male patients with metastatic cancer prior to chemotherapy. Cancer Res 1982;42:2495–2498. [PubMed] [Google Scholar]
- 4. Garcia JM, Li H, Mann D, Epner D, Hayes TG, Marcelli M, et al. Hypogonadism in male patients with cancer. Cancer 2006;106:2583–2591. [DOI] [PubMed] [Google Scholar]
- 5. Akamizu T, Kangawa K. Ghrelin for cachexia. J Cachexia Sarcopenia Muscle 2010;1:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hui D, Hannon BL, Zimmermann C, Bruera E. Improving patient and caregiver outcomes in oncology: team‐based, timely, and targeted palliative care. CA Cancer J Clin 2018;68:356–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Temel JS, Greer JA, Admane S, Gallagher ER, Jackson VA, Lynch TJ, et al. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non‐small‐cell lung cancer: results of a randomized study of early palliative care. J Clin Oncol 2011;29:2319–2326. [DOI] [PubMed] [Google Scholar]
- 8. Greer JA, Pirl WF, Jackson VA, Muzikansky A, Lennes IT, Heist RS, et al. Effect of early palliative care on chemotherapy use and end‐of‐life care in patients with metastatic non‐small‐cell lung cancer. J Clin Oncol 2012;30:394–400. [DOI] [PubMed] [Google Scholar]
- 9. Bakitas MA, Tosteson TD, Li Z, Lyons KD, Hull JG, Li Z, et al. Early versus delayed initiation of concurrent palliative oncology care: patient outcomes in the ENABLE III randomized controlled trial. J Clin Oncol 2015;33:1438–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Izumi K, Shigehara K, Nohara T, Narimoto K, Kadono Y, Nanjo S, et al. Androgen replacement therapy for cancer‐related symptoms in male advanced cancer patients: study protocol for a randomised prospective trial (ARTFORM study). J Med Invest 2017;64:202–204. [DOI] [PubMed] [Google Scholar]
- 11. Lunenfeld B, Saad F, Hoesl CE. ISA, ISSAM and EAU recommendations for the investigation, treatment and monitoring of late‐onset hypogonadism in males: scientific background and rationale. Aging Male 2005;8:59–74. [DOI] [PubMed] [Google Scholar]
- 12. Namiki M, Akaza H, Shimazui T, Ito N, Iwamoto T, Baba K, et al. Clinical practice manual for late‐onset hypogonadism syndrome. Int J Urol 2008;15:377–388. [DOI] [PubMed] [Google Scholar]
- 13. Suresh KP. An overview of randomization techniques: an unbiased assessment of outcome in clinical research. J Hum Reprod Sci 2011;4:8–11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14. Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 1991;7:6–9. [PubMed] [Google Scholar]
- 15. Ribaudo JM, Cella D, Hahn EA, Lloyd SR, Tchekmedyian NS, Von Roenn J, et al. Re‐validation and shortening of the Functional Assessment of Anorexia/Cachexia Therapy (FAACT) questionnaire. Qual Life Res 2000;9:1137–1146. [DOI] [PubMed] [Google Scholar]
- 16. Utech AE, Tadros EM, Hayes TG, Garcia JM. Predicting survival in cancer patients: the role of cachexia and hormonal, nutritional and inflammatory markers. J Cachexia Sarcopenia Muscle 2012;3:245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Costelli P, Carbo N, Tessitore L, Bagby GJ, Lopez‐Soriano FJ, Argilés JM, et al. Tumor necrosis factor‐alpha mediates changes in tissue protein turnover in a rat cancer cachexia model. J Clin Invest 1993;92:2783–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burkart M, Schieber M, Basu S, Shah P, Venugopal P, Borgia JA, et al. Evaluation of the impact of cachexia on clinical outcomes in aggressive lymphoma. Br J Haematol 2019;186:45–53. [DOI] [PubMed] [Google Scholar]
- 19. Vigano A, Piccioni M, Trutschnigg B, Hornby L, Chaudhury P, Kilgour R. Male hypogonadism associated with advanced cancer: a systematic review. Lancet Oncol 2010;11:679–684. [DOI] [PubMed] [Google Scholar]
- 20. Tenover JL. Male hormone replacement therapy including “andropause.”. Endocrinol Metab Clin North Am 1998;27:969–987. [DOI] [PubMed] [Google Scholar]
- 21. Taya M, Koh E, Izumi K, Iijima M, Maeda Y, Matsushita T, et al. Comparison of testosterone fractions between Framingham Heart Study participants and Japanese participants. Int J Urol 2014;21:689–695. [DOI] [PubMed] [Google Scholar]
- 22. Khosla S, Melton LJ III, Riggs BL. Clinical review 144: estrogen and the male skeleton. J Clin Endocrinol Metab 2002;87:1443–1450. [DOI] [PubMed] [Google Scholar]
- 23. Konaka H, Sugimoto K, Orikasa H, Iwamoto T, Takamura T, Takeda Y, et al. Effects of long‐term androgen replacement therapy on the physical and mental statuses of aging males with late‐onset hypogonadism: a multicenter randomized controlled trial in Japan (EARTH Study). Asian J Androl 2016;18:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barrett‐Connor E, Goodman‐Gruen D, Patay B. Endogenous sex hormones and cognitive function in older men. J Clin Endocrinol Metab 1999;84:3681–3685. [DOI] [PubMed] [Google Scholar]
- 25. Christiansen K, Knussmann R. Sex hormones and cognitive functioning in men. Neuropsychobiology 1987;18:27–36. [DOI] [PubMed] [Google Scholar]
- 26. Kloner RA, Carson C III, Dobs A, Kopecky S, Mohler ER III. Testosterone and cardiovascular disease. J Am Coll Cardiol 2016;67:545–557. [DOI] [PubMed] [Google Scholar]
- 27. Abi‐Ghanem C, Robison LS, Zuloaga KL. Androgens' effects on cerebrovascular function in health and disease. Biol Sex Differ 2020;11:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ahmed T, Alattar M, Pantalone K, Haque R. Is testosterone replacement safe in men with cardiovascular disease? Cureus 2020;12:e7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morales A, Bebb RA, Manjoo P, Assimakopoulos P, Axler J, Collier C, et al. Diagnosis and management of testosterone deficiency syndrome in men: clinical practice guideline. CMAJ 2015;187:1369–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Izumi K, Chang C. Targeting inflammatory cytokines‐androgen receptor (AR) signaling with ASC‐J9® to better battle prostate cancer progression. Onco Targets Ther 2013;2:e26853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fang LY, Izumi K, Lai KP, Liang L, Li L, Miyamoto H, et al. Infiltrating macrophages promote prostate tumorigenesis via modulating androgen receptor‐mediated CCL4‐STAT3 signaling. Cancer Res 2013;73:5633–5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Izumi K, Mizokami A, Lin WJ, Lai KP, Chang C. Androgen receptor roles in the development of benign prostate hyperplasia. Am J Pathol 2013;182:1942–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Del Fabbro E, Garcia JM, Dev R, Hui D, Williams J, Engineer D, et al. Testosterone replacement for fatigue in hypogonadal ambulatory males with advanced cancer: a preliminary double‐blind placebo‐controlled trial. Support Care Cancer 2013;21:2599–2607. [DOI] [PubMed] [Google Scholar]
- 34. Amiaz R, Seidman SN. Testosterone and depression in men. Curr Opin Endocrinol Diabetes Obes 2008;15:278–283. [DOI] [PubMed] [Google Scholar]
- 35. Walther A, Breidenstein J, Miller R. Association of testosterone treatment with alleviation of depressive symptoms in men: a systematic review and meta‐analysis. JAMA Psychiat 2019;76:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gould TD, Georgiou P, Brenner LA, Brundin L, Can A, Courtet P, et al. Animal models to improve our understanding and treatment of suicidal behavior. Transl Psychiatry 2017;7:e1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Carrier N, Saland SK, Duclot F, He H, Mercer R, Kabbaj M. The anxiolytic and antidepressant‐like effects of testosterone and estrogen in gonadectomized male rats. Biol Psychiatry 2015;78:259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wainwright SR, Workman JL, Tehrani A, Hamson DK, Chow C, Lieblich SE, et al. Testosterone has antidepressant‐like efficacy and facilitates imipramine‐induced neuroplasticity in male rats exposed to chronic unpredictable stress. Horm Behav 2016;79:58–69. [DOI] [PubMed] [Google Scholar]
- 39. Paizanis E, Hamon M, Lanfumey L. Hippocampal neurogenesis, depressive disorders, and antidepressant therapy. Neural Plast 2007;2007:73754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Daly L, Dolan R, Power D, Ní Bhuachalla É, Sim W, Fallon M, et al. The relationship between the BMI‐adjusted weight loss grading system and quality of life in patients with incurable cancer. J Cachexia Sarcopenia Muscle 2020;11:160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dolan RD, Daly L, Sim WMJ, Fallon M, Ryan A, McMillan DC, et al. Comparison of the prognostic value of ECOG‐PS, mGPS and BMI/WL: implications for a clinically important framework in the assessment and treatment of advanced cancer. Clin Nutr 2019. pii: S0261–5614;33212–33211. [DOI] [PubMed] [Google Scholar]
- 42. Kasprzak A. The role of tumor microenvironment cells in colorectal cancer (CRC) cachexia. Int J Mol Sci 2021;22:1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ma J, Wu CF, Wang F, Yang JY, Dong YX, Su GY, et al. Neurological mechanism of Xiaochaihutang's antidepressant‐like effects to socially isolated adult rats. J Pharm Pharmacol 2016;68:1340–1349. [DOI] [PubMed] [Google Scholar]
- 44. Lorigooini Z, Boroujeni SN, Sayyadi‐Shahraki M, Rahimi‐Madiseh M, Bijad E, Amini‐Khoei H. Limonene through attenuation of neuroinflammation and nitrite level exerts antidepressant‐like effect on mouse model of maternal separation stress. Behav Neurol 2021;2021:8817309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang C, Sui G, Li D, Wang L, Zhang S, Lei P, et al. Exogenous IGF‐1 alleviates depression‐like behavior and hippocampal mitochondrial dysfunction in high‐fat diet mice. Physiol Behav 2021;229:113236. [DOI] [PubMed] [Google Scholar]
- 46. Adachi S, Tokuda N, Kobayashi Y, Tanaka H, Sawai H, Shibahara H, et al. Association between the serum insulin‐like growth factor‐1 concentration in the first trimester of pregnancy and postpartum depression. Psychiatry Clin Neurosci 2021; 10.1111/pcn.13200 Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barbonetti A, D'Andrea S, Francavilla S. Testosterone replacement therapy. Andrology 2020;8:1551–1566. [DOI] [PubMed] [Google Scholar]
- 48. Nieschlag E, Cüppers HJ, Wiegelmann W, Wickings EJ. Bioavailability and LH‐suppressing effect of different testosterone preparations in normal and hypogonadal men. Horm Res 1976;7:138–145. [DOI] [PubMed] [Google Scholar]
- 49. Dev R, Del Fabbro E, Dalal S. Endocrinopathies and cancer cachexia. Curr Opin Support Palliat Care 2019;13:286–291. [DOI] [PubMed] [Google Scholar]
- 50. Kato Y, Shigehara K, Nakashima K, Iijima M, Kawagushi S, Nohara T, et al. The five‐year effects of testosterone replacement therapy on lipid profile and glucose tolerance among hypogonadal men in Japan: a case control study. Aging Male 2020;23:23–28. [DOI] [PubMed] [Google Scholar]
- 51. Corona G, Torres LO, Maggi M. Testosterone therapy: what we have learned from trials. J Sex Med 2020;17:447–460. [DOI] [PubMed] [Google Scholar]
- 52. Arver S, Luong B, Fraschke A, Ghatnekar O, Stanisic S, Gultyev D, et al. Is testosterone replacement therapy in males with hypogonadism cost‐effective? An analysis in Sweden. J Sex Med 2014;11:262–272. [DOI] [PubMed] [Google Scholar]
- 53. Ponce OJ, Spencer‐Bonilla G, Alvarez‐Villalobos N, Serrano V, Singh‐Ospina N, Rodriguez‐Gutierrez R, et al. The efficacy and adverse events of testosterone replacement therapy in hypogonadal men: a systematic review and meta‐analysis of randomized, placebo‐controlled trials. J Clin Endocrinol Metab 2018;103:1745–1754. [DOI] [PubMed] [Google Scholar]
- 54. Izumi K, Shigehara K, Nohara T, Narimoto K, Kadono Y, Mizokami A. Both high and low serum total testosterone levels indicate poor prognosis in patients with prostate cancer. Anticancer Res 2017;37:5559–5564. [DOI] [PubMed] [Google Scholar]
- 55. Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, et al. Testosterone therapy in men with hypogonadism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2018;103:1715–1744. [DOI] [PubMed] [Google Scholar]
- 56. Mulhall JP, Trost LW, Brannigan RE, Kurtz EG, Redmon JB, Chiles KA, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol 2018;200:423–432. [DOI] [PubMed] [Google Scholar]
- 57. Hamauchi S, Furuse J, Takano T, Munemoto Y, Furuya K, Baba H, et al. A multicenter, open‐label, single‐arm study of anamorelin (ONO‐7643) in advanced gastrointestinal cancer patients with cancer cachexia. Cancer 2019;125:4294–4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Baracos VE, Mazurak VC, Bhullar AS. Cancer cachexia is defined by an ongoing loss of skeletal muscle mass. Ann Palliat Med 2019;8:3–12. [DOI] [PubMed] [Google Scholar]
- 59. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Average ESAS scores, and FAACT subscale and total scores at each time point.


