Abstract
Background
Wasting is a common complication of kidney failure that leads to weight loss and poor outcomes. Recent experimental data identified parathyroid hormone (PTH) as a driver of adipose tissue browning and wasting, but little is known about the relations among secondary hyperparathyroidism, weight loss, and risk of mortality in dialysis patients.
Methods
We included 42,319 chronic in‐centre haemodialysis patients from the Dialysis Outcomes and Practice Patterns Study phases 2–6 (2002–2018). Linear mixed models were used to estimate the association between baseline PTH and percent weight change over 12 months, adjusting for country, demographics, comorbidities, and labs. Accelerated failure time models were used to assess 12 month weight loss as a mediator between baseline high PTH and mortality after 12 months.
Results
Baseline PTH was inversely associated with 12 month weight change: 12 month weight loss >5% was observed in 21%, 18%, 18%, 17%, 15%, and 14% of patients for PTH ≥600 pg/mL, 450–600, 300–450, 150–300, 50–150, and <50 pg/mL, respectively. In adjusted analyses, 12 month weight change compared with PTH 150–299 pg/mL was −0.60%, −0.12%, −0.10%, +0.15%, and +0.35% for PTH ≥600, 450–600, 300–450, 50–150, and <50 pg/mL, respectively. This relationship was robust regardless of recent hospitalization and was more pronounced in persons with preserved appetite. During follow‐up after the 12 month weight measure [median, 1.0 (interquartile range, 0.6–1.7) years; 6125 deaths], patients with baseline PTH ≥600 pg/mL had 11% [95% confidence interval (CI), 9–13%] shorter lifespan, and 18% (95% CI, 14–23%) of this effect was mediated through weight loss ≥2.5%.
Conclusions
Secondary hyperparathyroidism may be a novel mechanism of wasting, corroborating experimental data, and, among chronic dialysis patients, this pathway may be a mediator between elevated PTH levels and mortality. Future research should determine whether PTH‐lowering therapy can limit weight loss and improve longer term dialysis outcomes.
Keywords: Haemodialysis, Mortality, Secondary hyperparathyroidism, Weight loss
Introduction
Wasting is a syndrome characterized by loss of fat and skeletal muscle that accompanies many chronic diseases including kidney failure. 1 , 2 , 3 Observational studies have shown strong associations between signs of wasting, such as weight loss, and mortality risk in patients undergoing haemodialysis. 4 , 5 Individuals with kidney failure may consume less food due to dietary restrictions or loss of appetite, 6 , 7 , 8 , 9 but wasting is quite different from malnutrition caused by insufficient food intake. 2 , 3 One key characteristic of wasting is elevated basal energy expenditure, which leads to loss of adipose tissue and skeletal muscle through enhanced fat and protein catabolism. 2 , 3 Patients with kidney failure have been shown to have increased resting energy expenditure particularly in the presence of comorbidities. 10 , 11 , 12
While the aetiology of wasting in kidney failure may be multifactorial, 2 , 3 recent experimental studies have demonstrated that parathyroid hormone (PTH) induces a phenotypic switch from white to brown adipocytes (a phenomenon termed adipose tissue browning) and thereby drives thermogenesis and hypermetabolism. 13 , 14 Secondary hyperparathyroidism (SHPT) is a common complication in haemodialysis patients. 15 , 16 , 17 However, few studies have examined the potential link between SHPT and wasting in haemodialysis patients. 18 , 19 , 20 Furthermore, SHPT has been shown to be associated with increased risk of death, 16 , 17 but the causal effect of SHPT on mortality is uncertain and underlying mechanisms not well understood.
We analysed data from the Dialysis Outcomes and Practice Patterns Study (DOPPS) to test the hypotheses that (i) SHPT leads to weight loss in haemodialysis patients and (ii) this pathway in part mediates the association of SHPT with mortality.
Materials and methods
Patients and data collection
The DOPPS (www.dopps.org) is an international prospective cohort study of patients 18 years or older treated with in‐centre haemodialysis. Details on study design and methods have been published. 21 , 22 Briefly, the study population was composed of randomly selected patients from a random sample of dialysis facilities within each country. Data for demographics, comorbid conditions, laboratory values, and prescriptions were abstracted from medical records using uniform and standardized data collection tools. Lack of appetite 23 was collected in patient questionnaire (‘During the past four weeks, to what extent were you bothered by lack of appetite?’) at baseline in Phases 2–3, and annually in Phases 4–6. Mortality and hospitalization events were collected during study follow‐up. Study approval was obtained by a central institutional review board within each DOPPS country. Additional study approvals and patient consent were obtained as required by national and local ethics committee regulations.
The current analysis included 42 319 DOPPS participants from Phase 2 (2002–2004) through Phase 6 (2015–2018) in order to sample a wide distribution of PTH levels. Further details of the study sample are shown in Figure 1 . Country‐specific sample sizes in 12 month weight loss analysis are shown in Supporting Information, Table S1. Study patients had baseline PTH and post‐dialysis weight at least 1 year after haemodialysis initiation, and post‐dialysis weight measured 12 months later. PTH levels and weight changes commonly experienced by patients soon after initiating haemodialysis 24 were not the subject of this study. Participants were recruited through 792 unique facilities from North America (the United States and Canada), Japan, Europe (Belgium, France, Germany, Italy, Spain, Sweden, and the United Kingdom), Australia, New Zealand, Russia, the Gulf Cooperation Council (Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, and United Arab Emirates), Turkey, and China. Turkey and China were only included in the 12 month weight loss analysis, while the other countries were included in all analysis.
Figure 1.
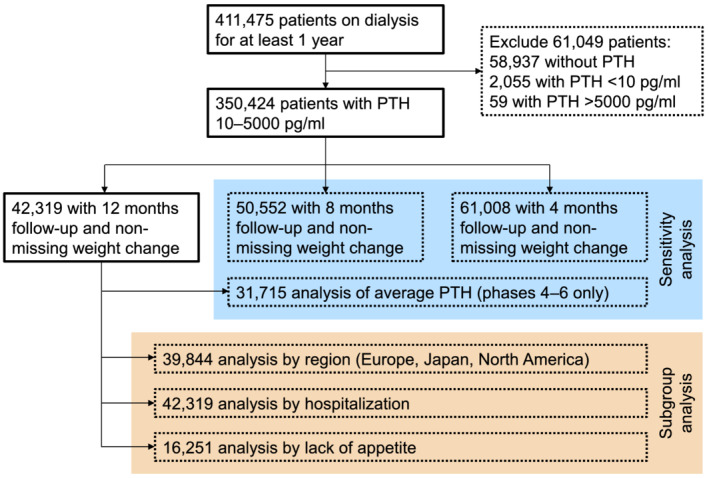
Study flow chart. PTH, parathyroid hormone.
Individuals enrolled in multiple study phases were treated as independent observations and may be represented multiple times. Weight loss was defined as the 12 month weight minus the baseline weight. Patients with absolute or percent weight changes greater than the 99th percentile or less than the first percentile, within country (absolute weight change smaller than −20 to −9 kg or bigger than 6 to 14 kg; percent weight change smaller than −26% to −14% or bigger than 11% to 22%, depending on country), were excluded from all analyses.
For the majority of the study period, intact PTH assays were the only assay type available for clinical practice, and <10% of DOPPS facilities reported using bio‐intact (or whole) assays. PTH values from facilities reporting use of bio‐intact assays were multiplied by 1.7 to obtain an equivalent intact PTH value. 25 , 26 Missing or unknown assay types were assumed to be intact assays.
Statistical analyses
Standard descriptive statistics were used to compare demographics and clinical characteristics of the study population according to baseline PTH levels. We used linear mixed models to estimate the association of baseline PTH levels with the percent weight change over 12 months. Random intercepts were included for each facility to account for potential clustering of outcomes by facility. We used multivariable models to incrementally adjust for potential confounding. Model 1 adjusted for country, study phase, and electronic health record data source (US Phases 4–6 only). Model 2 further adjusted for age, sex, time on dialysis, 13 comorbid conditions (as listed in Table 1 ), single‐pool Kt/V, and dry weight. Model 3 further adjusted for albumin, haemoglobin, creatinine, calcium, and phosphorus. Model 4 further adjusted for calcium‐based binder, sevelamer, lanthanum, other phosphate binders, active vitamin D derivatives, and calcimimetics. We used Model 3 as the base model and Model 4 as a sensitivity analysis. Patients in the PTH category of 150–299 pg/mL were used as the analysis reference group. The percent weight change values were compared with the mean for this group when presenting results in figures. As the association between PTH and percent weight change may have differed geographically, we performed a stratified analysis by region (restricted to Europe, Japan, and North America). Similar stratified analyses based on body weight tertiles in each region, or based on lack of appetite were also performed. Additionally, because hospitalization may lead to marked weight loss, we separately examined the association between PTH and weight loss over 12 months among patients who were and were not hospitalized during this period.
Table 1.
Baseline characteristics by PTH categories
| Variable | PTH (pg/mL) | |||||
|---|---|---|---|---|---|---|
| <50 | 50–149 | 150–299 | 300–449 | 450–599 | ≥600 | |
| (n = 3169) | (n = 9035) | (n = 12 500) | (n = 7228) | (n = 4024) | (n = 6363) | |
| Demographics | ||||||
| Age, mean (SD), years | 64.0 (13.4) | 65.3 (13.5) | 65.1 (13.7) | 63.6 (14.4) | 61.5 (14.6) | 57.2 (15.3) |
| Male, % | 57.4 | 58.6 | 59.0 | 57.5 | 56.4 | 54.6 |
| Time on dialysis, mean (SD), years | 6.6 (7.1) | 5.5 (6.0) | 4.9 (5.1) | 5.0 (5.1) | 5.3 (5.2) | 5.9 (5.1) |
| Dry weight, mean (SD), kg | 65.1 (18.8) | 68.4 (19.8) | 74.2 (21.1) | 77.8 (21.9) | 79.1 (22.4) | 79.3 (23.3) |
| Single‐pool Kt/V, mean (SD) | 1.50 (0.29) | 1.52 (0.30) | 1.55 (0.29) | 1.56 (0.28) | 1.56 (0.28) | 1.55 (0.28) |
| Comorbidities, % | ||||||
| Coronary artery disease | 35.3 | 36.3 | 34.2 | 32.3 | 31.0 | 28.2 |
| Congestive heart failure | 25.1 | 25.7 | 27.1 | 24.8 | 27.4 | 25.0 |
| Cerebrovascular disease | 15.0 | 14.7 | 12.0 | 11.3 | 10.6 | 9.0 |
| Peripheral vascular disease | 21.3 | 22.7 | 22.5 | 22.1 | 21.2 | 18.0 |
| Other cardiovascular disease | 30.3 | 29.7 | 26.1 | 23.3 | 23.5 | 20.9 |
| Hypertension | 79.7 | 82.2 | 82.6 | 83.4 | 83.3 | 82.0 |
| Diabetes | 38.7 | 44.8 | 51.3 | 53.6 | 50.7 | 43.2 |
| Neurologic disease | 9.7 | 8.9 | 8.5 | 8.2 | 7.7 | 8.6 |
| Psychiatric disease | 13.1 | 14.4 | 16.5 | 17.9 | 19.7 | 19.6 |
| Lung disease | 8.2 | 9.7 | 9.8 | 9.3 | 9.5 | 8.3 |
| Cancer | 12.9 | 11.5 | 10.7 | 9.2 | 8.7 | 7.4 |
| Gastrointestinal bleeding | 4.5 | 4.3 | 4.2 | 4.5 | 4.6 | 3.9 |
| Recurrent cellulitis | 6.0 | 6.9 | 7.0 | 6.6 | 7.3 | 6.2 |
| Laboratory tests | ||||||
| Albumin, mean (SD), g/dL | 3.7 (0.5) | 3.7 (0.5) | 3.8 (0.4) | 3.8 (0.4) | 3.8 (0.4) | 3.8 (0.4) |
| Haemoglobin, mean (SD), g/dL | 11.0 (1.4) | 11.1 (1.4) | 11.2 (1.3) | 11.1 (1.3) | 11.1 (1.4) | 11.1 (1.4) |
| Creatinine, mean (SD), mg/dL | 9.4 (3.0) | 8.9 (3.0) | 8.7 (2.9) | 8.9 (2.9) | 9.2 (2.9) | 10.0 (3.1) |
| Calcium, mean (SD), mg/dL | 9.2 (0.9) | 9.1 (0.8) | 9.0 (0.7) | 9.0 (0.8) | 9.0 (0.8) | 9.0 (0.8) |
| Phosphorus, mean (SD), mg/dL | 5.1 (1.6) | 5.0 (1.5) | 5.1 (1.5) | 5.3 (1.5) | 5.5 (1.6) | 6.0 (1.8) |
| Total cholesterol, mean (SD), mg/dL | 160 (39) | 157 (40) | 156 (42) | 154 (41) | 154 (40) | 156 (41) |
| Medication, % | ||||||
| ESA | 88.5 | 88.3 | 87.4 | 86.6 | 86.5 | 86.8 |
| Active vitamin D derivatives | ||||||
| IV | 12.8 | 24.3 | 39.8 | 49.5 | 51.9 | 48.5 |
| Oral | 35.4 | 29.9 | 26.7 | 26.6 | 28.4 | 26.6 |
| Cinacalcet | 6.0 | 11.0 | 13.4 | 17.3 | 21.4 | 32.3 |
| Phosphate binder | ||||||
| Calcium‐based | 68.9 | 59.0 | 50.0 | 45.5 | 44.9 | 43.6 |
| Lanthanum | 7.2 | 9.4 | 7.7 | 7.3 | 7.4 | 7.2 |
| Sevelamer | 20.6 | 25.8 | 33.5 | 38.9 | 40.6 | 43.9 |
| Other | 6.6 | 7.3 | 7.8 | 9.3 | 10.2 | 12.2 |
| Lack of appetite, % a | ||||||
| Not at all | 57.2 | 55.2 | 56.7 | 55.9 | 53.5 | 54.1 |
| Somewhat | 24.9 | 25.2 | 24.0 | 23.3 | 23.6 | 24.1 |
| Moderately/very much/extremely | 17.9 | 19.6 | 19.3 | 20.8 | 23.0 | 21.7 |
Abbreviations: ESA, erythropoiesis‐stimulating agent; IV, intravenous; PTH, parathyroid hormone.
Data on lack of appetite were available in 16 251 patients.
For mortality analysis, follow‐up time began at the end of the 12 month observation period of weight loss. That is, analysis included the period between baseline plus 12 months, until the first event of loss to follow‐up, transplantation, end of study phase, death, the most recent date of data availability within each study phase, or 7 days after leaving the facility due to transfer or change in kidney replacement therapy modality. To assess weight loss as a mediator between high PTH and mortality, the cut points for PTH and weight loss were chosen based on Cox models adjusting for covariates in Model 3 listed above, stratified by country, and accounting for facility clustering using robust sandwich covariance estimators. We then used accelerated failure time models with a generalized gamma‐distributed baseline to model patient survival (or relative life expectancy) from the baseline, in a manner similar to that described by VanderWeele. 27 Errors in the coefficient estimates for model parameters and mediation effects were computed using a bootstrap approach (200 iterations). 28 Total and direct effects of high PTH on mortality and the proportion mediated were reported. Figure S1 provides an illustration of the mediation analysis pathways between PTH, weight loss, and mortality (panel A), and the timing of the data used in the three major models for the mediation analysis (panel B).
As a sensitivity analysis, we estimated the association between average PTH levels during 4 months and the percent weight change during the subsequent 12 months: this analysis included only participants from Phases 4–6, because monthly laboratory values were not available in earlier phases. In addition, we based the analysis on patients with 4 and 8 month weight measurements after a baseline weight and PTH and estimated the association between baseline PTH and 4 or 8 month weight change. We also tested 4 or 8 month weight changes as mediators between PTH and mortality.
The proportion of missing data was <10% for all variables, except for single‐pool Kt/V (18%). We constructed a multiple‐imputation data set with 10 replicates using IVEware 29 ; models were estimated separately for each replicate, and results were combined in standard fashion according to Rubin's rules. 30 While it was used in the imputation process for other variables, lack of appetite was not imputed, due to concerns that some of the patients may have not answered the patient questionnaire because they were too sick to do so, and these patients may have non‐representative appetite scores even after adjustment. Patients with missing appetite information were thus excluded in the appetite subgroup analysis. All analyses were performed using SAS software, Version 9.4 (SAS Institute Inc).
Results
Patient characteristics
Among 42 319 patients at baseline, the mean (SD) body weight was 74 (22) kg, and the median PTH level was 251 pg/mL (interquartile range, 131–444 pg/mL, Table S2). Patients with higher PTH tended to be younger; have higher body weight, fewer comorbid conditions, and higher serum phosphorus levels; and be more often prescribed sevelamer, intravenous active vitamin D, and cinacalcet (Table 1 ). Among patients with data on lack of appetite (n = 16 251), the degree of appetite loss was comparable across categories of PTH.
Parathyroid hormone and weight change
Overall, the mean percent change in body weight for 12 months was −0.67%. Weight loss was more common than weight gain—11% of patients gained more than 5% weight, while 17% had more than 5% weight loss. Baseline PTH was inversely associated with 12 month weight change: mean 12 month weight change was −1.02%, −0.68%, −0.72%, −0.66%, −0.52%, and −0.34% for PTH ≥600, 450–600, 300–450, 50–150, and <50 pg/mL, respectively; the corresponding proportion of patients with 12 month weight loss >5% was 21%, 18%, 18%, 17%, 15%, and 14%, respectively (Figure 2 ).
Figure 2.
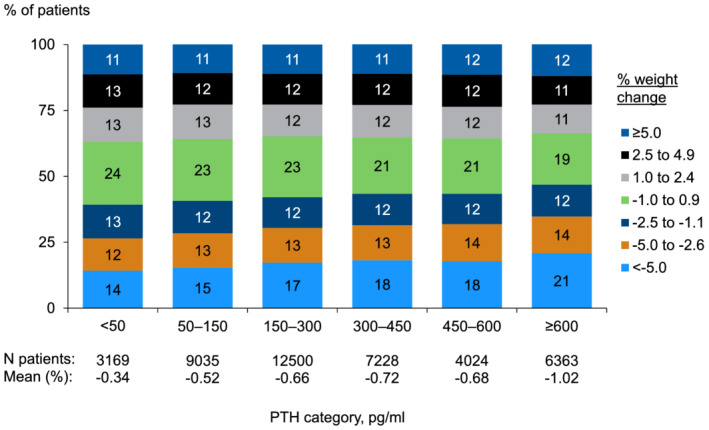
Distribution of 12 month percent weight change by baseline PTH level. PTH, parathyroid hormone.
Adjusted models also found a relationship between higher PTH and weight loss: 12 month weight change compared with PTH 150–299 pg/mL was −0.60%, −0.12%, −0.10%, +0.15%, and +0.34% for PTH ≥600, 450–600, 300–450, 50–150, and <50 pg/mL, respectively (Figure 3 , Model 3). The results were consistent across different models.
Figure 3.

Association of baseline PTH with 12‐month percent weight change. PTH, parathyroid hormone; Ref, reference. Model 1 adjusted for country, study phase, and electronic health record data source (US Phases 4–6 only), accounting for facility clustering. Model 2 adjusted for covariates in Model 1 plus age, sex, time on dialysis, 13 comorbid conditions, single‐pool Kt/V, and dry weight. Model 3 adjusted for covariates in Model 2 plus albumin, haemoglobin, creatinine, calcium, and phosphorus. Model 4 adjusted for covariates in Model 3 plus calcium‐based binder, sevelamer, lanthanum, other phosphate binders, active vitamin D derivatives, and calcimimetics. The P value for trend was <0.001 for each model. The mean actual weight change was shown for the reference group, and other groups were plotted relative to the reference group based on adjusted model results.
The association between PTH and percent weight change was most dramatic in North America (P for interaction between PTH and region for outcome percent weight change, <0.001), where patients with PTH ≥600 pg/mL lost 0.65% [95% confidence interval (CI) −0.88%, −0.41%] more weight compared with those with PTH 150–299 pg/mL (Figure 4 ). Patients with lower body weight had slightly larger percent weight changes, but direction of the associations between PTH and percent weight change were consistent across body weight tertiles in North America and Europe (Figure S2).
Figure 4.

Association of baseline PTH with 12‐month percent weight change, by region. PTH, parathyroid hormone; Ref, reference. Model adjusted for country, study phase, electronic health record data source (US Phases 4–6 only), age, sex, time on dialysis, 13 comorbid conditions, single‐pool Kt/V, dry weight, albumin, haemoglobin, creatinine, calcium, and phosphorus, accounting for facility clustering. The P value for trend was <0.001 for Europe and north American, and 0.004 for Japan. The mean actual weight change was shown for the reference group, and other groups were plotted relative to the reference group based on adjusted model results.
The mean percent weight loss was 1.56% for patients who were hospitalized during 12 months (n = 17 918) and 0.02% for those who were not hospitalized (n = 24 401). Regardless of whether patients were hospitalized or not, PTH was associated with percent weight change (Figure 5 ). We also performed stratified analyses by lack of appetite in available samples. While patients with poor appetite showed larger percent weight changes, high PTH was associated with weight loss only in persons who endorsed preserved appetite (Figure 6 , P for interaction between PTH and lack of appetite for outcome percent weight change, 0.06).
Figure 5.

Association of baseline PTH with 12 month percent weight change in patients who were hospitalized and those who were not. PTH, parathyroid hormone; Ref, reference. Model adjusted for country, study phase, electronic health record data source (US Phases 4–6 only), age, sex, time on dialysis, 13 comorbid conditions, single‐pool Kt/V, dry weight, albumin, haemoglobin, creatinine, calcium, and phosphorus, accounting for facility clustering. The P value for trend was <0.001 for each analysis. The mean actual weight change was shown for the reference group, and other groups were plotted relative to the reference group based on adjusted model results.
Figure 6.

Association of baseline PTH with 12 month percent weight change, by lack of appetite. PTH, parathyroid hormone; Ref, reference. Model adjusted for country, study phase, electronic health record data source (US Phases 4–6 only), age, sex, time on dialysis, 13 comorbid conditions, single‐pool Kt/V, dry weight, albumin, haemoglobin, creatinine, calcium, and phosphorus, accounting for facility clustering. The analysis includes only individuals with available data on lack of appetite question (n = 16 251). The five possible responses to this question were as follows: not at all, somewhat, moderately, very much, and extremely. Categories of moderately, very much, and extremely were combined into a single category due to the small sample size. The P value for trend was 0.44 for very much, 0.11 for somewhat, and <0.001 for not at all. The mean actual weight change was shown for the reference group, and other groups were plotted relative to the reference group based on adjusted model results.
Assessment of weight loss as mediator between elevated parathyroid hormone and mortality
The association between percent weight change in 12 months and mortality suggests that the risk of mortality was higher for those with weight loss ≥2.5% than for any other group (Figure S3). For this reason, we used an indicator variable for weight loss ≥2.5% in the mediation analyses. For similar reasons (Figure S4), we used an indicator variable for PTH ≥600 pg/mL.
For analyses based on the weight change of 12 month measurement period, 14% (6125/42303) of patients died during median follow‐up of 1.0 years (interquartile range, 0.6–1.7 years). Among patients who survived at least 12 months after the PTH measurement, those with PTH ≥600 pg/mL had an 11% (95% CI, 8–12%) shorter lifespan, and 18% (95% CI, 14–23%) of this effect was mediated through weight loss ≥2.5%.
Sensitivity analysis
When we used average PTH levels during 4 months as the exposure in participants from Phases 4–6 (n = 31 715), the association between PTH and percent weight change was qualitatively unchanged (Figures S5–S7). When we analysed percent weight change during 4 and 8 months (n = 61 008 and n = 50 552, respectively), the mean percent change in body weight for 4 and 8 months was −0.21% and −0.37%, respectively, compared with −0.67% for 12 months (Figure S8). In the mediation analysis, the indirect effect of PTH ≥600 pg/mL through the ≥2.5% weight loss over the 4 month period was 3% (95% CI 2–4%) of the 15% shorter lifespan during follow‐up. The indirect effect of PTH ≥600 pg/mL through the ≥2.5% weight loss over the 8‐month period was 11% (95% CI 9–13%) of the 14% shorter lifespan (Figure 7 ).
Figure 7.
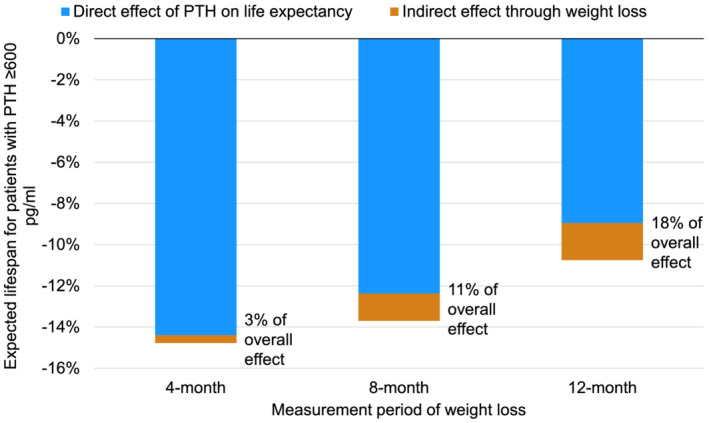
Association of baseline PTH ≥600 pg/mL with expected lifespan during post‐measurement follow‐up, mediated by weight loss ≥2.5% of body weight. PTH, parathyroid hormone. Model adjusted for country, study phase, electronic health record data source (US Phases 4–6 only), age, sex, time on dialysis, 13 comorbid conditions, single‐pool Kt/V, dry weight, albumin, haemoglobin, creatinine, calcium, and phosphorus, accounting for facility clustering.
Discussion
In this international DOPPS cohort of over 42 000 haemodialysis patients, we found that elevated PTH was associated with subsequent weight loss, a hallmark of wasting. This relationship was sustained after adjustment for numerous covariates, was robust regardless of whether patients were hospitalized or not, and was more pronounced in persons with preserved appetite. Furthermore, the association between PTH and weight loss partly mediated the higher risk of mortality associated with elevated PTH levels. These findings suggest that SHPT may be a novel mechanism of wasting and that this pathway may be a mediator between elevated PTH levels and mortality among maintenance dialysis patients.
Wasting is a common feature of kidney failure. A recent meta‐analysis reported that approximately 28–54% of maintenance dialysis patients show evidence of wasting. 31 Using data from the international DOPPS, we validate that weight loss, an important indicator of wasting, frequently occurs in this population: 31% of patients showed weight loss ≥2.5% during 12 months and 17% showed more than 5% weight loss. Such weight loss is a serious problem as it leads to poor clinical outcomes and increased mortality. 4 , 5 Our findings on the relationship between elevated PTH and weight loss support recent experimental evidence suggesting a direct role of PTH in adipose tissue browning 13 , 14 and indicate that targeting this pathway may hold promise for ameliorating wasting. Whether PTH‐lowering therapy can halt weight loss in haemodialysis patients with SHPT is important and worthy of further clinical research.
To our knowledge, this study is the first to show an association between elevated PTH and weight loss in a large cohort of haemodialysis patients. Our findings are consistent with a previous small study of haemodialysis patients showing that individuals with severe SHPT had increased resting energy expenditure. 19 Similar findings have also been reported in patients with primary hyperparathyroidism 13 , 14 and cancer patients with detectable levels of PTH‐related protein, a tumour‐derived factor that shares the same receptor with PTH. 32 , 33 Our results extend these findings and further confirm that the association between PTH and weight loss persisted even after adjustment for potential confounders, an important approach that has not been systematically evaluated. The consistent findings across the diverse clinical settings support the key role of the PTH/PTH‐related protein pathway in the pathogenesis of wasting.
While we speculate that the strong association between PTH and weight loss is a clinical consequence of the direct effects of PTH on adipose tissue, other factors may have also been implicated. Because high PTH is associated with the risk of fracture 16 , 34 , 35 and cardiovascular disease, 16 , 17 one may argue that the association between PTH and weight loss could be explained by such clinical events. However, the association persisted even when the analysis was restricted to patients who were not hospitalized. One could also postulate that the association between PTH and weight loss may simply reflect regression to the mean, 36 given that patients with higher PTH tended to have higher body weight at baseline. However, this possibility is also unlikely because the association was sustained after adjustment for baseline body weight and was consistent across subgroups stratified by baseline body weight. Finally, it should be noted that we did not have a direct measure of fluid status, so we cannot exclude the possibility that the observed greater weight loss among persons with high baseline PTH levels resulted from greater reduction in extracellular fluid volume. However, the present analysis excluded those who started haemodialysis within 1 year, during which volume overload is actively corrected. Furthermore, patients with higher PTH levels have been shown to be more likely to miss haemodialysis treatments, 37 suggesting poor patient adherence, which should rather lead to weight gain due to fluid retention through increased water and salt intake. 38
Interestingly, we observed a stronger association between PTH and weight loss in North America. By contrast, the association was less pronounced in Japan, where there was almost no association between PTH levels ≥300 pg/mL and weight loss. These contrasting findings may be explained by the difference in the management of SHPT. A prior DOPPS analysis 17 reported a steady increase over time in PTH levels in North America after the introduction of the international guideline suggesting a higher PTH target than previously recommended, 39 while PTH levels remained stable in Japan where the local guideline recommends a much lower PTH target. 40 Thus, patients with elevated PTH levels would be likely to receive pharmacotherapy to lower PTH levels or parathyroidectomy more quickly in Japan, which might attenuate the association between elevated baseline PTH and weight loss over subsequent months.
We also observed that the relationship between PTH and weight loss almost disappeared in patients with poor appetite. Loss of appetite is common in kidney failure patients and is associated with insufficient food intake, poor nutritional status, and inflammation. 6 , 7 , 8 Importantly, inflammatory cytokines also contribute to adipose tissue browning and increased energy expenditure. 41 As such, patients with poor appetite have multiple factors that lead to weight loss, obscuring the effect of PTH on weight loss that may occur through adipose tissue browning. Alternatively, there might be indirect mechanisms through which certain factors related to appetite loss attenuate the biological action of PTH on adipose tissue. Additional studies are needed to explore these possibilities.
Secondary hyperparathyroidism has been associated with increased risk of death. 16 , 17 In the Evaluation of Cinacalcet Hydrochloride Therapy to Lower Cardiovascular Events trial, cinacalcet did not significantly reduce the risk of death, but analyses adjusted for baseline covariates or accounting for study‐drug exposure showed a significant effect. 42 However, the mechanisms underlying these observations have not been fully elucidated. One potential explanation is vascular calcification. However, PTH may not directly induce arterial calcification, 43 and the associations between PTH and mortality have been independent of serum calcium and phosphorus, 16 , 17 potent inducers of calcification. 44 Another possibility is fracture events, because high PTH levels are associated with fracture risk, 16 , 34 , 35 and these events lead to a marked increase in subsequent mortality. 45 However, the rates of fractures requiring hospitalization are approximately six‐fold lower than that of mortality in the DOPPS, 45 indicating that fracture events could explain at most only a small fraction of the increased mortality with elevated PTH. In our study, we found by mediation analysis that weight loss accounted for 18% of the association between PTH and mortality. These findings highlight the possibility that weight loss is a clinically relevant pathway linking SHPT and mortality. It should be however noted that the analysis cannot infer causality, because the pathways of association were based on observational data and sensitive to assumptions inherent to mediation analysis. 27 Future studies are needed to test whether weight loss contributes to the relationship between SHPT and mortality.
Our study has additional limitations. First, we lacked data on food intake. There is a possibility that food intake differs by PTH levels. However, elevated PTH remained to be associated with weight loss in an analysis restricted to individuals with preserved appetite. Furthermore, patients with higher PTH were younger and had higher body weight and fewer comorbidities, indicating a likelihood of higher food intake. If so, this should counteract the effects of PTH on weight loss. Of note, we did not use normalized protein catabolic rate as a surrogate of dietary protein intake, because it also reflects endogenous protein catabolism 46 and thus could be on the causal pathway between SHPT and weight loss. Second, we evaluated weight loss for up to 12 months, which is a relatively short period. However, given the observed increasing magnitude of weight loss during the 12 month period and the progressive nature of SHPT, 15 we expect that the association of SHPT with weight loss will be yet more pronounced over longer follow‐up. Third, we did not account for changes in PTH levels during longitudinal follow‐up. However, these levels remain relatively stable over time in the majority of the DOPPS participants, 47 and we confirmed that the association between baseline PTH and subsequent weight loss was constant over time. Finally, multiple assays are available for measurement of PTH. Although the majority of DOPPS facilities reported using intact assays and we converted bio‐intact assay measurements into equivalent intact PTH values, 25 , 26 there may be large inter‐assay variability in PTH results 48 and inter‐individual variability in the ratio of bio‐intact/intact PTH. 49 Nonetheless, this variation would bias the association of PTH with weight loss toward the null. Strengths of our study include a large sample size, international data capture with a standardized protocol, prospective study design, and detailed covariate data that enabled us to control for potential confounders.
In conclusion, using data from the international DOPPS cohort, we showed that elevated PTH was associated with subsequent weight loss and this pathway partly mediated the association between elevated PTH levels and mortality in maintenance dialysis patients. These findings offer new evidence in support of a possible role of SHPT in the pathogenesis of wasting and weight loss. Future research should determine whether PTH‐lowering therapy can limit or prevent weight loss and improve longer‐term dialysis outcomes.
Conflict of interest
H.K. has received honoraria, consulting fees, and/or grant support from Bayer Yakuhin, Chugai Pharmaceutical, Japan Tobacco, Kyowa Kirin, Novartis, and Ono Pharmaceutical. S.Y. has received honoraria from Kyowa Kirin. T.N. is an employee of Kyowa Kirin. P.E. has received honoraria, consulting fees, and/or grant support from Amgen, Sanofi, and Medice. J.Z., D.S.F., K.P.M., E.W.Y., and B.M.R. are employees for the non‐profit research organization Arbor Research Collaborative for Health, which has designed and carries out the DOPPS Program. B.M.R. has received consultancy fees or travel reimbursement from AstraZeneca, GlaxoSmithKline, and Kyowa Kirin, all paid directly to his institution of employment. M.F. has received honoraria, consulting fees, and/or grant support from Bayer Yakuhin, Fresenius Kabi, Kissei Pharmaceutical, Kyowa Kirin, Ono Pharmaceutical, and Torii Pharmaceutical. The remaining authors declare that they have no other relevant financial interests.
Supporting information
Table S1. Number of patients included in 12‐month weight loss analysis by phase and country
Table S2. Distribution of baseline PTH by region
Figure S1. Illustration of mediation analysis. (A) Pathways between baseline PTH, weight loss, and mortality. (B) Timing of data used in three major models
Figure S2. Association of baseline PTH with 12‐month percent weight change, by body weight in Europe (A), Japan (B) and North America (C)
Figure S3. Association between percent weight change and mortality
Figure S4. Association between baseline PTH and mortality
Figure S5. Distribution of 12‐month percent weight change by baseline mean PTH level
Figure S6. Association of baseline mean PTH with 12‐month percent weight change
Figure S7. Association of baseline mean PTH with 12‐month percent weight change, by region
Figure S8. Association of baseline PTH with percent weight change in 4, 8, and 12 months
Acknowledgements
This manuscript was directly supported by Kyowa Kirin. Global support for ongoing DOPPS Programs is provided without restriction on publications by a variety of funders (details in https://www.dopps.org/AboutUs/Support.aspx). All grants were made to Arbor Research Collaborative for Health and not to co‐authors directly. None of the funders had any role in study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit this report for publication. The authors certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. 50
Komaba H., Zhao J., Yamamoto S., Nomura T., Fuller D. S., McCullough K. P., Evenepoel P., Christensson A., Zhao X., Alrukhaimi M., Al‐Ali F., Young E. W., Robinson B. M., and Fukagawa M. (2021) Secondary hyperparathyroidism, weight loss, and longer term mortality in haemodialysis patients: results from the DOPPS, Journal of Cachexia, Sarcopenia and Muscle, 12, 855–865, 10.1002/jcsm.12722
References
- 1. Fouque D, Kalantar‐Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, et al. A proposed nomenclature and diagnostic criteria for protein‐energy wasting in acute and chronic kidney disease. Kidney Int 2008;73:391–398. [DOI] [PubMed] [Google Scholar]
- 2. Mak RH, Ikizler AT, Kovesdy CP, Raj DS, Stenvinkel P, Kalantar‐Zadeh K. Wasting in chronic kidney disease. J Cachexia Sarcopenia Muscle 2011;2:9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carrero JJ, Stenvinkel P, Cuppari L, Ikizler TA, Kalantar‐Zadeh K, Kaysen G, et al. Etiology of the protein‐energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J Ren Nutr 2013;23:77–90. [DOI] [PubMed] [Google Scholar]
- 4. Pifer TB, McCullough KP, Port FK, Goodkin DA, Maroni BJ, Held PJ, et al. Mortality risk in hemodialysis patients and changes in nutritional indicators: DOPPS. Kidney Int 2002;62:2238–2245. [DOI] [PubMed] [Google Scholar]
- 5. Kalantar‐Zadeh K, Kopple JD, Kilpatrick RD, McAllister CJ, Shinaberger CS, Gjertson DW, et al. Association of morbid obesity and weight change over time with cardiovascular survival in hemodialysis population. Am J Kidney Dis 2005;46:489–500. [DOI] [PubMed] [Google Scholar]
- 6. Kalantar‐Zadeh K, Block G, McAllister CJ, Humphreys MH, Kopple JD. Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am J Clin Nutr 2004;80:299–307. [DOI] [PubMed] [Google Scholar]
- 7. Burrowes JD, Larive B, Chertow GM, Cockram DB, Dwyer JT, Greene T, et al. Self‐reported appetite, hospitalization and death in haemodialysis patients: findings from the Hemodialysis (HEMO) study. Nephrol Dial Transplant 2005;20:2765–2774. [DOI] [PubMed] [Google Scholar]
- 8. Lopes AA, Elder SJ, Ginsberg N, Andreucci VE, Cruz JM, Fukuhara S, et al. Lack of appetite in haemodialysis patients—associations with patient characteristics, indicators of nutritional status and outcomes in the international DOPPS. Nephrol Dial Transplant 2007;22:3538–3546. [DOI] [PubMed] [Google Scholar]
- 9. Ikizler TA, Cano NJ, Franch H, Fouque D, Himmelfarb J, Kalantar‐Zadeh K, et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int 2013;84:1096–1107. [DOI] [PubMed] [Google Scholar]
- 10. Ikizler TA, Wingard RL, Sun M, Harvell J, Parker RA, Hakim RM. Increased energy expenditure in hemodialysis patients. J Am Soc Nephrol 1996;7:2646–2653. [DOI] [PubMed] [Google Scholar]
- 11. Wang AY, Sea MM, Tang N, Sanderson JE, Lui S‐F, Li PK‐T, et al. Resting energy expenditure and subsequent mortality risk in peritoneal dialysis patients. J Am Soc Nephrol 2004;15:3134–3143. [DOI] [PubMed] [Google Scholar]
- 12. Avesani CM, Kamimura MA, Cuppari L. Energy expenditure in chronic kidney disease patients. J Ren Nutr 2011;21:27–30. [DOI] [PubMed] [Google Scholar]
- 13. Kir S, Komaba H, Garcia AP, Economopoulos KP, Liu W, Lanske B, et al. PTH/PTHrP receptor mediates cachexia in models of kidney failure and cancer. Cell Metab 2016;23:315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. He Y, Liu RX, Zhu MT, Shen W‐B, Xie J, Zhang Z‐Y, et al. The browning of white adipose tissue and body weight loss in primary hyperparathyroidism. EBioMedicine 2019;40:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Drüeke TB. Cell biology of parathyroid gland hyperplasia in chronic renal failure. J Am Soc Nephrol 2000;11:1141–1152. [DOI] [PubMed] [Google Scholar]
- 16. Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004;15:2208–2218. [DOI] [PubMed] [Google Scholar]
- 17. Tentori F, Wang M, Bieber BA, Karaboyas A, Li Y, Jacobson SH, et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol 2015;10:98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chou FF, Lee CH, Chen JB. General weakness as an indication for parathyroid surgery in patients with secondary hyperparathyroidism. Arch Surg 1999;134:1108–1111. [DOI] [PubMed] [Google Scholar]
- 19. Cuppari L, de Carvalho AB, Avesani CM, Kamimura MA, Dos Santos Lobão RR, Draibe SA. Increased resting energy expenditure in hemodialysis patients with severe hyperparathyroidism. J Am Soc Nephrol 2004;15:2933–2939. [DOI] [PubMed] [Google Scholar]
- 20. Komaba H, Fukagawa M. Secondary hyperparathyroidism and protein‐energy wasting in end‐stage renal disease. Ther Apher Dial 2018;22:246–250. [DOI] [PubMed] [Google Scholar]
- 21. Young EW, Goodkin DA, Mapes DL, Port FK, Keen ML, Chen K, et al. The dialysis outcomes and practice patterns study (DOPPS): an international hemodialysis study. Kidney Int 2000;57:S74–S81. [Google Scholar]
- 22. Pisoni RL, Gillespie BW, Dickinson DM, Chen K, Kutner MH, Wolfe RA. The dialysis outcomes and practice patterns study (DOPPS): design, data elements, and methodology. Am J Kidney Dis 2004;44:S7–S15. [DOI] [PubMed] [Google Scholar]
- 23. Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB. Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res 1994;3:329–338. [DOI] [PubMed] [Google Scholar]
- 24. Melamed ML, Eustace JA, Plantinga L, Jaar BG, Fink NE, Coresh J, et al. Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: a longitudinal study. Kidney Int 2006;70:351–357. [DOI] [PubMed] [Google Scholar]
- 25. Gao P, Scheibel S, D'Amour P, John MR, Rao SD, Schmidt‐Gayk H, et al. Development of a novel immunoradiometric assay exclusively for biologically active whole parathyroid hormone 1‐84: implications for improvement of accurate assessment of parathyroid function. J Bone Miner Res 2001;16:605–614. [DOI] [PubMed] [Google Scholar]
- 26. Kazama JJ, Omori T, Ei I, Ei K, Oda M, Maruyama H, et al. Circulating 1‐84 PTH and large C‐terminal PTH fragment levels in uremia. Clin Exp Nephrol 2003;7:144–149. [DOI] [PubMed] [Google Scholar]
- 27. VanderWeele T. Explanation in Causal Inference: Methods for Mediation and Interaction. New York, NY: Oxford University Press; 2015. [Google Scholar]
- 28. Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods 2008;40:879–891. [DOI] [PubMed] [Google Scholar]
- 29. Raghunathan TE, Solenberger PW, Van Hoewyk J. IVEware: Imputation and Variance Estimation Software: User Guide. Ann Arbor, MI: Institute for Social Research, University of Michigan; 2002. [Google Scholar]
- 30. Little RJA, Rubin DB. Statistical Analysis With Missing Data. New York, NY: Wiley; 1987. [Google Scholar]
- 31. Carrero JJ, Thomas F, Nagy K, Arogundade F, Avesani CM, Chan M, et al. Global prevalence of protein‐energy wasting in kidney disease: a meta‐analysis of contemporary observational studies from the International Society of Renal Nutrition and Metabolism. J Ren Nutr 2018;28:380–392. [DOI] [PubMed] [Google Scholar]
- 32. Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, et al. Tumour‐derived PTH‐related protein triggers adipose tissue browning and cancer cachexia. Nature 2014;513:100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hong N, Yoon HJ, Lee YH, Kim HR, Lee BW, Rhee Y, et al. Serum PTHrP predicts weight loss in cancer patients independent of hypercalcemia, inflammation, and tumor burden. J Clin Endocrinol Metab 2016;101:1207–1214. [DOI] [PubMed] [Google Scholar]
- 34. Danese MD, Kim J, Doan QV, Dylan M, Griffiths R, Chertow GM. PTH and the risks for hip, vertebral, and pelvic fractures among patients on dialysis. Am J Kidney Dis 2006;47:149–156. [DOI] [PubMed] [Google Scholar]
- 35. Jadoul M, Albert JM, Akiba T, Akizawa T, Arab L, Bragg‐Gresham JL, et al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the dialysis outcomes and practice patterns study. Kidney Int 2006;70:1358–1366. [DOI] [PubMed] [Google Scholar]
- 36. Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol 2005;34:215–220. [DOI] [PubMed] [Google Scholar]
- 37. Al Salmi I, Larkina M, Wang M, Subramanian L, Morgenstern H, Jacobson SH, et al. Missed hemodialysis treatments: international variation, predictors, and outcomes in the dialysis outcomes and practice patterns study (DOPPS). Am J Kidney Dis 2018. Nov;72:634–643. [DOI] [PubMed] [Google Scholar]
- 38. Saran R, Bragg‐Gresham JL, Rayner HC, Goodkin DA, Keen ML, Van Dijk PC, et al. Nonadherence in hemodialysis: associations with mortality, hospitalization, and practice patterns in the DOPPS. Kidney Int 2003. Jul;64:254–262. [DOI] [PubMed] [Google Scholar]
- 39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD‐MBD Work Group . KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease‐mineral and bone disorder (CKD‐MBD). Kidney Int. 2009;76:S1–S130. [DOI] [PubMed] [Google Scholar]
- 40. Fukagawa M, Yokoyama K, Koiwa F, Taniguchi M, Shoji T, Kazama JJ, et al. Clinical practice guideline for the management of chronic kidney disease‐mineral and bone disorder. Ther Apher Dial 2013;17:247–288. [DOI] [PubMed] [Google Scholar]
- 41. Petruzzelli M, Schweiger M, Schreiber R, Campos‐Olivas R, Tsoli M, Allen J, et al. A switch from white to brown fat increases energy expenditure in cancer‐associated cachexia. Cell Metab 2014;20:433–447. [DOI] [PubMed] [Google Scholar]
- 42. EVOLVE Trial Investigators . Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med 2012;367:2482–2494. [DOI] [PubMed] [Google Scholar]
- 43. Lomashvili K, Garg P, O'Neill WC. Chemical and hormonal determinants of vascular calcification in vitro. Kidney Int 2006;69:1464–1470. [DOI] [PubMed] [Google Scholar]
- 44. Shanahan CM, Crouthamel MH, Kapustin A, Giachelli CM. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ Res 2011;109:697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tentori F, McCullough K, Kilpatrick RD, McCullough K, Kilpatrick RD, Bradbury BD, et al. High rates of death and hospitalization follow bone fracture among hemodialysis patients. Kidney Int 2014;85:166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. National Kidney Foundation . K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis 2000;35:S1–S140. [DOI] [PubMed] [Google Scholar]
- 47. Yamamoto S, Karaboyas A, Komaba H, Taniguchi M, Nomura T, Bieber BA, et al. Mineral and bone disorder management in hemodialysis patients: comparing PTH control practices in Japan with Europe and North America: the dialysis outcomes and practice patterns study (DOPPS). BMC Nephrol 2018;19:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Souberbielle JC, Boutten A, Carlier MC, Chevenne D, Coumaros G, Lawson‐Body E, et al. Inter‐method variability in PTH measurement: implication for the care of CKD patients. Kidney Int 2006;70:345–350. [DOI] [PubMed] [Google Scholar]
- 49. Melamed ML, Eustace JA, Plantinga LC, Jaar BG, Fink NE, Parekh RS, et al. Third‐generation parathyroid hormone assays and all‐cause mortality in incident dialysis patients: the CHOICE study. Nephrol Dial Transplant 2008;23:1650–1658. [DOI] [PubMed] [Google Scholar]
- 50. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Number of patients included in 12‐month weight loss analysis by phase and country
Table S2. Distribution of baseline PTH by region
Figure S1. Illustration of mediation analysis. (A) Pathways between baseline PTH, weight loss, and mortality. (B) Timing of data used in three major models
Figure S2. Association of baseline PTH with 12‐month percent weight change, by body weight in Europe (A), Japan (B) and North America (C)
Figure S3. Association between percent weight change and mortality
Figure S4. Association between baseline PTH and mortality
Figure S5. Distribution of 12‐month percent weight change by baseline mean PTH level
Figure S6. Association of baseline mean PTH with 12‐month percent weight change
Figure S7. Association of baseline mean PTH with 12‐month percent weight change, by region
Figure S8. Association of baseline PTH with percent weight change in 4, 8, and 12 months


