Abstract
One of the current myopathies affecting the chicken meat industry is deep pectoral myopathy (DPM), also known as green muscle disease or Oregon disease, the condition is considered a major problem in poultry processing lines. Thus, the present study proposes to examine the meat quality of the Pectoralis major muscle (breast fillet) from carcasses of broilers affected by DPM in Pectoralis minor muscle (tender) and from a control group. Breast fillets samples were harvested from Ross AP95 broilers that were slaughtered at 42 days of age and were selected to the occurrence of the myopathy (score 2 and score 3) and from a control group without (score 0) the presence of myopathy. Chemical composition, cholesterol, fatty acid profile, pH, color, water-holding capacity, cooking loss, shear force, sarcomere length, and collagen were analyzed in the breast fillet. And with the results it was observed the samples classified as DPM score 2 had a higher moisture and a lower protein percentage. The higher values lipid was found in the samples from broilers affected by DPM. There was no difference (P > 0.05) fatty acid profile only to C10:0, C15:0, C17:0, C20:0, and C18:2c9,t11. Differences were detected (P < 0.05) for the pH, WHC, SF, and sarcomere length of the samples from broilers affected by DPM. The higher pH observed in the samples from birds classified as DPM score 2 and the higher WHC values were observed in the samples affected by DPM (score 2 and 3). The SF (P < 0.05) among samples, with the most tender samples (lower SF values) being those unaffected by the condition DPM (score 0). The dorsal side surface, where the Pectoralis major muscle is in contact with the Pectoralis minor muscle, higher L* values were found in the meat affected by the myopathy. Although deep pectoral myopathy affects the Pectoralis minor muscle of broilers, it can also alter the qualitative characteristics and chemical composition of the breast fillets (Pectoralis major muscle).
Key words: broiler, meat, Pectoralis major, Pectoralis minor, quality
INTRODUCTION
The forecast for global chicken meat production is revised marginally lower to 100.0 million tons as declines in the United States and China are more than offset by growth in Brazil and the EU. Despite a dampening in the outlook, world production in 2020 remains higher (nearly 1%) vs. last year. Demand will be relatively resilient as consumers pursue lower priced animal protein in the face of an economic downturn (USDA, 2020). The poultry sector continues to grow and industrialize in many parts of the world as driven by the growing population, increased purchasing power, and urbanization.
In the last decades, consumers have exhibited a greater preference for chicken meat over other types of muscle foods. This increased demand has intensified broiler production, which in turn resulted in the emergence of myopathies associated with chicken meat. As a consequence, the poultry industry has shown a growing interest in understanding the impacts of such abnormalities on the various traits related to meat quality.
One of the current myopathies affecting the chicken meat industry is deep pectoral myopathy (DPM), also known as green muscle disease or Oregon disease. The condition is considered a major problem in poultry processing lines. Though not recent, the disease has become increasingly frequent in the flesh of broilers and turkeys selected for the production of carcasses with a larger proportion of breast muscle. The myopathy can cause significant commercial losses to producers, since the lesions are identified only after the carcass is deboned and may interfere with product quality.
Deep pectoral myopathy is characterized by necrosis and atrophy of the Pectoralis minor or supracoracoideus muscle (tender), which exhibits variations in color, ranging from a pinkish hemorrhage-like appearance to a grayish-green discoloration (Bilgili and Hess, 2008; Kijowskia and Kupińska, 2013).
The myopathy considerably affects the appearance of tender meat but is not associated with infectious agents (Bilgili and Hess, 2008). Its occurrence may be related to factors such as genetics, sex, slaughter weight, bird mobility, and rearing conditions (Kijowski and Kupińska, 2013).
Once DPM is detected in Pectoralis minor muscle, the affected part must be removed, and the rest of the carcass may still be suitable for human consumption. However, the cutting process performed around the affected region damages the product and generates economic losses to the industry, which is mainly because it affects the carcass part of greatest commercial value (Petracci and Cavani, 2012).
Deep pectoral myopathy has some similarities in the microscopic lesions seen in the Pectoralis minor muscle (Wight and Siller, 1980) with that of white striping (WS) and woody breast (WB), which suggests that ischemia may also be associated with these modern myopathies (Kuttappan et al., 2016).
Little information exists on the quality of breast meat from broilers affected by DPM. Thus, the present study proposes to examine the meat quality of the Pectoralis major muscle (breast fillet) from carcasses of broilers affected by DPM in Pectoralis minor or supracoracoideus muscle (tender) and from a control group.
MATERIALS AND METHODS
Sample Collection
A total of 120 samples were selected at 3 h postmortem from a commercial broiler slaughter plant in the south region of Brazil following the procedures adopted by the processing plant.
Were collected from Ross AP95 male broilers that were slaughtered at 42 d. For the classification step, breast meat samples, Pectoralis major muscle with Pectoralis minor muscle together were selected at random on the slaughter line according to the occurrence of the myopathy in the Pectoralis minor muscle (tender), DPM score 2 (n = 40), DPM score 3 (n = 40) and from a control group without the presence of myopathy, unaffected by DPM, score 0 (n = 40). The breast meat samples were classified as to the degree of severity of DPM in the Pectoralis minor muscle before the deboning operation, following the criterion of condemnation due to special diseases, which establishes that only the affected part of the carcass should be discarded (Brasil, 1997). The breast samples collected did not present other myopathies as WS and wooden breast, were separated only with DPM in Pectoralis minor muscle in conjunction with the Pectoralis major muscle.
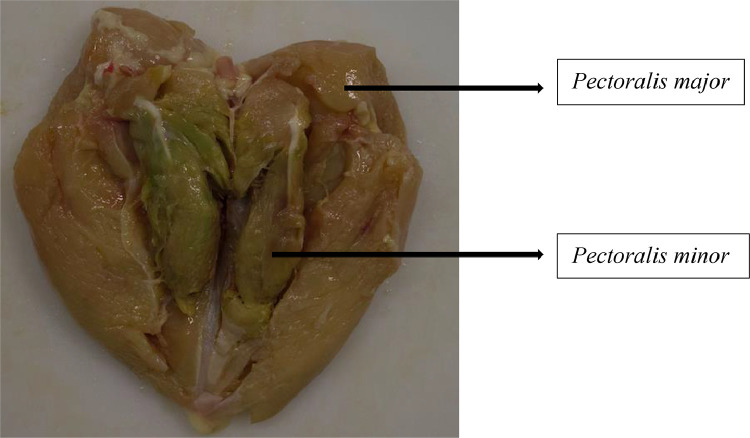
In accordance with the methodology adopted by Bilgili and Hess (2008), samples exhibiting well-defined lesions on the Pectoralis minor (tender) muscle were classified as DPM score 2, with lesions surrounded by a clear hemorrhagic ring. Those which showed progressive degeneration of the Pectoralis minor muscle, with the damaged muscle tissue having a greenish appearance, were classified as DPM score 3. Both classifications were performed on the samples with Pectoralis major muscle and Pectoralis minor muscle together (Figure 1).
Figure 1.
Breast meat samples (Pectoralis major + Pectoralis minor muscle) with deep pectoral myopathy (DPM), score 3. Source: The author.
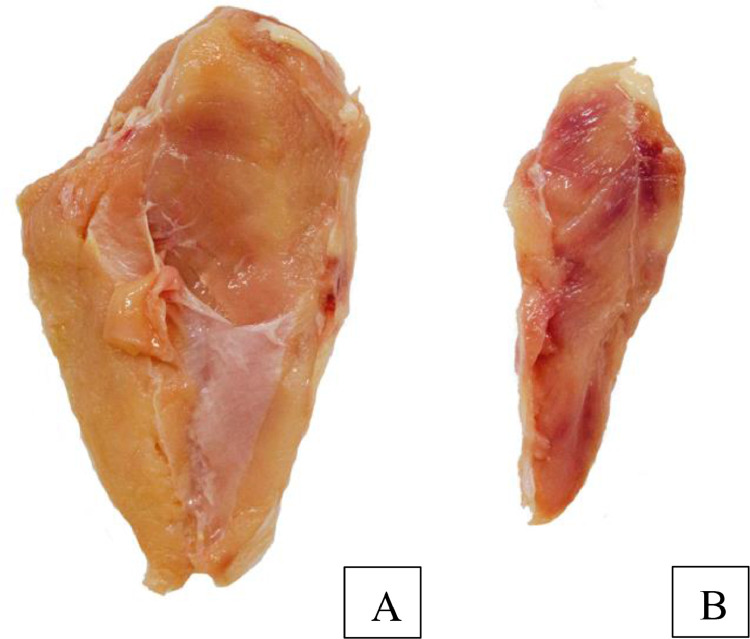
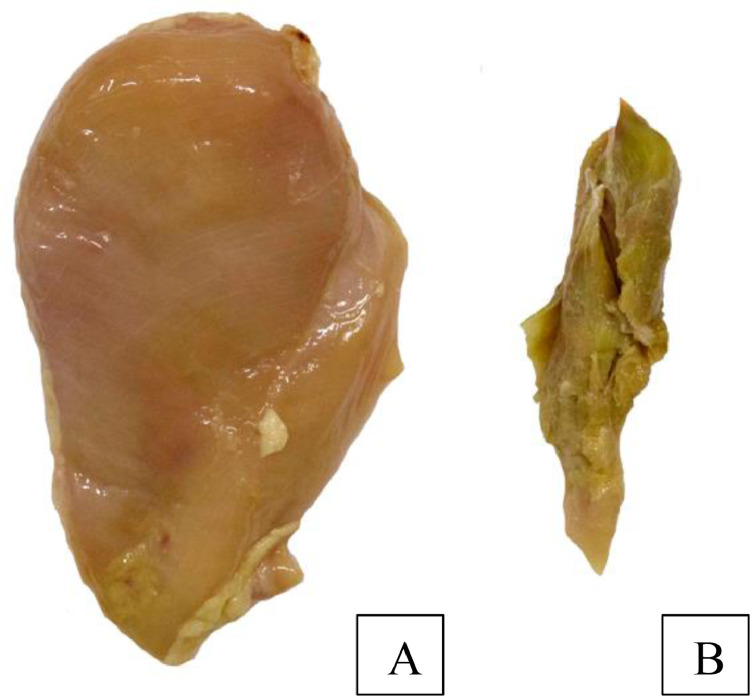
After the classification step, the Pectoralis minor muscle (Figures 2 and 3) of each was discarded and samples of the remaining Pectoralis major muscle were sent for quality analysis. Were performed all quality analyses meat in Pectoralis major muscle (breast fillet) and the proposed physical analyses were performed immediately after collection. The breast fillet samples to be later used for the chemical analysis were frozen at –20 °C for a period no longer than 30 d.
Figure 2.
The Pectoralis major muscle (A) used for quality analysis and Pectoralis minor muscle with deep pectoral myopathy (DPM) with score 2 (B) discarded. Source: The author.
Figure 3.
The Pectoralis major muscle (A) used for quality analysis and Pectoralis minor muscle with deep pectoral myopathy (DPM) with score 3 (B) discarded. Source: The author.
Laboratory Analyses
Procedures recommended by the Association of Official Analytical Chemists (AOAC, 2005) were performed to determine the chemical composition of the breast fillets (Pectoralis major muscle), including moisture, protein, and ash contents (AOAC methods 950.46, 977.14 and 920.153, respectively). The lipid content was determined by the methodology described by Bligh and Dyer (1959).
Cholesterol was measured by an adapted version of the methodology described by Saldanha et al. (2004), using a 0.5-g sample of lyophilized rather than a fresh sample (the standard curve was adapted as a function of the sample's dry matter). The cholesterol content was measured using an enzymatic kit for blood cholesterol analysis (Labtest: COLESTEROL Liquiform, Ref.: 76 MS: 10009010068). Readings were performed using a spectrophotometer (Shimadzu UV-1800, Kyoto, Japan), with λ defined as indicated by the manufacturer of the enzymatic kit (500 nm).
Fatty acids were isolated from samples according to the method proposed by Bligh and Dyer (1959), which extracts the lipid phase from the sample. The fatty acid esterification was carried out according to the method proposed by Maia and Rodriguez-Amaya (1993) using a gas chromatograph (Shimadzu 14B, Shimadzu Corporation, Kyoto, Japan) equipped with a flame ionization detector and a fused silica capillary column (Omegawax 250); H2 was used as the carrier gas. The identification of peaks was made by comparison with the retention times of standards with known composition.
The pH was measured in duplicate by inserting the penetration electrode of a digital pH meter (Testo 205, Testo Inc., Sparta, NJ) directly into the cranial part of the breast fillets (Pectoralis major muscle). Water-holding capacity (WHC) was determined by the method proposed by Hamm (1961). For this assessment, a 2-g sample was placed between 2 filter papers and acrylic plates and a 10-kg weight was then placed on top of the plate for five minutes. The sample was weighed again to determine the amount of water lost, which was calculated using the following formula: WHC = Final weight × 100 / Initial weight.
To evaluate cooking loss (CL), samples of similar weights and sizes were weighed individually, vacuum-packed and cooked in a water bath (85°C) for 30 min. Next, the samples were cooled at room temperature and weighed again to determine CL, which was calculated by the following formula: CL = (Initial weight – Final weight) × 100/Initial weight (Honikel, 1987).
Shear force (SF) was analyzed in samples which had been used for the analysis of CL, by the Meullenet-Owens Razor Shear (MORS) and/or Blunt-MORS method (Cavitt et al., 2004; Lee et al., 2008). The instrument was coupled to a texture analyzer (TA-XT2i, Stable Micro Systems, Godalming, UK). Samples were cut with the fibers perpendicular to the direction of the MORS (crosshead speed of 10 mm/s, sample shear depth of 20 mm and trigger force of 0.1 N). The force required to shear the samples was expressed in Newtons (N).
Sarcomere length was determined by phase-contrast microscopy (Cross et al., 1981). A 0.5-g sample was homogenized with 30 mL of a 50:50 KCl (0.08 mol/L) and KI (0.08 mol/L) solution in a Turrax homogenizer (MA 102, Marconi Equipamentos para Laboratório Ltd., Piracicaba, Brazil) at a speed higher than 15,000 rpm, for 30 s, to rupture the cells and facilitate the removal of myofibrils for suspension. Subsequently, the homogenate was deposited on a microscope slide, which was then covered with a coverslip. The material was then read using a phase-contrast microscope (Novel BM2100) under 1,000× magnification (100× objective, 10× ocular and these images generated by the phase-contrast microscope were measured in micrometers (μm).
Color was determined in the ventral (skin side) and dorsal (bone side, in contact with the Pectoralis minor muscle) surfaces of the Pectoralis major muscle, both at 3 points (cranial, middle, and caudal regions) and the 3 averages of each were used. Meat color was determined using a colorimeter (Minolta Chrome Meter CR-400, Konica Minolta Sensing, Inc., Osaka, Japan), which employs the CIELAB system (lightness [L*], red intensity [a*], and yellow intensity [b*]).
The concentrations of total, soluble and insoluble collagens were quantified by measuring the amino acid hydroxyproline, as proposed by Woessner Junior (1961) and Cross et al. (1973) and adapted by Hadlich et al. (2006). Five grams of samples were weighed and frozen in 50-mL falcon tubes to which 20 mL distilled water were later added. Subsequently, the tubes were placed in a water bath (80°C) for 2 h. Next, the samples were homogenized in an Ultra-turrax homogenizer (MA 102, Marconi Equipamentos para Laboratório Ltd., Piracicaba, Brazil) (22,000 rpm) for 1 min and centrifuged (HITACHI CR22N, Japan) (4,000 rpm) for 15 min. The samples were transferred to autoclavable tubes and separated into sediment and supernatant. Thirty milliliters of 6N HCl were added to the supernatant and 50 mL 6N HCl were added to the sediment (Woessner Junior, 1961). The samples were hydrolyzed in an autoclave for 4 h (120°C, 1 atm) (Cross et al., 1973). The pH was adjusted to 6.0 using 2N NaOH. Thereafter, the samples were filtered in volumetric flasks (250 and 100 mL for sediment and supernatant, respectively), whose volumes were then completed with distilled water. A 10-mL aliquot was transferred to new volumetric flasks (100 and 50 mL for sediment and supernatant, respectively), whose volumes were also completed with distilled water. Next, 2-mL aliquots were pipetted into test tubes, to which 1 mL of an oxidation reagent (Chloramine-T 1.41%) and 1 mL of a color reagent (10 g p-Dimethylaminobenzaldehyde in 35 mL 60% perchloric acid and 65 mL isopropanol) were subsequently added. The tubes containing the samples were kept in a water bath (60°C) for 15 min and the solution was read in a spectrophotometer (Shimadzu UV-1800, Kyoto, Japan) at λ = 560 nm. The collagen content was estimated as 7.14 times the hydroxyproline content (Hadlich et al., 2006).
Statistical Analysis
The experiment was set up as a completely randomized design with 3 treatments (one control group – unaffected by DPM, score 0; and degrees of severity – DPM scores 2 and 3) of 40 replicates each, in total 120 samples. Data were analyzed using the One-Way ANOVA procedure of SAS (2002) software (Statistical Analysis System; SAS Institute Inc, Cary, NC). Results were subjected to analysis of variance, and, in case of significance, means were compared by Tukey's test with significance defined as P < 0.05.
RESULTS AND DISCUSSION
There was a difference (P < 0.05) for the percentages of moisture, protein, and lipid (Table 1) between the groups. The breast fillets (Pectoralis major muscle) from samples classified as DPM score 2 showed a higher moisture percentage and a lower protein percentage. Lower protein percentages were also observed by Yalcin et al. (2018) in breast samples classified as DPM score 2 in study the occurrence of deep pectoral myopathy in broilers.
Table 1.
Means and standard deviations of chemical components of Pectoralis major muscle (breast fillets) samples obtained from broilers affected by deep pectoral myopathy (DPM).
| DPM Score |
||||
|---|---|---|---|---|
| Composition (%) | 0 | 2 | 3 | P-value |
| Moisture | 70.59 ± 0.80B | 71.59 ± 0.37A | 70.93 ± 0.39B | 0.0016 |
| Protein | 25.75 ± 1.82A | 22.65 ± 1.95B | 26.80 ± 3.53A | 0.0005 |
| Lipid | 2.19 ± 0.26B | 2.28 ± 0.47A | 2.32 ± 0.56A | 0.0412 |
| Ash | 1.75 ± 0.41A | 1.65 ± 0.46A | 1.83 ± 0.38A | 0.6214 |
| Cholesterol (mg/100 g) | 106.25 ± 6.06A | 105.26 ± 8.04A | 109.23 ± 4.11A | 0.3530 |
Pectoralis major muscle associated with Pectoralis minor muscle with DPM score 0: unaffected, score 2: muscles with coagulative necrosis, fibrous tissue texture and necrotic area and score 3: with the damaged muscle tissue having a greenish appearance.
Means followed by distinct letters (in the columns) differ by Tukey's test (P < 0.05).
The lower protein content found in the meat samples affected by DPM score 2 may indicate muscle fiber degeneration and further studies are needed to note that the presence of myopathies causes changes in the integrity of the muscle fiber and how this process occurs in DPM. Stangierski et al. (2019) characterized deep pectoral myopathy in breast fillets and, reported unfavorable changes in the histological structure of fillet muscle fibers and the native state of proteins. Especially myosin, may have caused the redistribution of water, in limited migration of free water molecules, which may have been caused by structural changes in proteins.
Kuttappan et al. (2012), in study the occurrence of WS in broiler breast fillets which resulted in more space for fat deposition. However, the regenerative capacity of satellite cells is finite; if the muscle loses its regenerative capacity, muscle tissue is replaced by adipose or connective tissue (Daughtry et al., 2017). In this study, although the samples are only from DPM, absent from other myopathies, it was also verified higher values lipid were found in the breast meat samples from broilers affected by the myopathy in the Pectoralis minor muscle.
Experiments on muscle degenerations have shown that muscles with white striping and wooden breast have lower protein and ash contents (Kuttappan et al., 2013; Mazzoni et al., 2015; Petracci et al., 2015). The same was reported by Yalcin et al. (2018) in a study on DPM and lower protein percentages in breast fillets this study was observed too.
Anatomopathological aspects of DPM in broiler and reported the presence of muscle lesions in both the Pectoralis minor (tender) and the Pectoralis major (though at a lower intensity) muscles, with presence of fibrovascular tissue and foci of regeneration in the area peripheral to the lesions (Vieira et al., 2006). Thus, the damaged fibers were probably replaced with adipose tissue, during the process of regeneration of the injured tissue, which may explain the increased lipid content and changes chemical components of the breast fillets (Pectoralis major muscle).
There was no difference (P > 0.05) fatty acid profile only to C10:0, C15:0, C17:0, C20:0, and C18:2c9,t11. The fatty acid profile C20:5n3 and C22:6n3 are considered to be important for human health and there was difference (P < 0.05) between unaffected meats from myopathy and the affected group (Table 2).
Table 2.
Mean values and standard deviation of fatty acid profile of Pectoralis major muscle samples affected by deep pectoral myopathy (DPM) in the Pectoralis minor.
| DPM Score |
||||
|---|---|---|---|---|
| Fatty acid | 0 | 2 | 3 | P-value |
| C10:0 | 0.000 | 0.000 | 0.000 | 0.0500 |
| C12:0 | 0.000B | 0.013A | 0.003AB | 0.0202 |
| C14:0 | 0.283B | 0.380A | 0.383A | 0.0003 |
| C15:0 | 0.083 | 0.076 | 0.078 | 0.5801 |
| C16:0 | 22.001B | 23.060A | 23.210A | 0.0083 |
| C17:0 | 0.130 | 0.135 | 0.125 | 0.5229 |
| C18:0 | 11.561A | 8.043B | 9.595B | 0.0069 |
| C20:0 | 0.076 | 0.085 | 0.081 | 0.1144 |
| Total SFA | 56.501A | 31.792B | 33.475B | 0.0005 |
| C14:1 | 0.030B | 0.058A | 0.055A | 0.0050 |
| C16:1 | 1.258B | 2.121A | 2.198A | 0.0094 |
| C 17:1 | 0.125A | 0.050B | 0.038B | 0.0006 |
| C18:1n9c | 20.913B | 28.798A | 25.592A | 0.0005 |
| C18:1n7 | 2.758A | 1.798B | 2.758A | <0.001 |
| C20:1n9 | 0.285A | 0.260AB | 0.238B | 0.0455 |
| Total MUFA | 25.369B | 33.085A | 30.879A | 0.0006 |
| C18:2n6c | 19.535B | 27.758A | 25.422A | 0.0001 |
| C18:2c9,t11 | 0.015 | 0.011 | 0.013 | 0.8752 |
| C20:2 | 1.245A | 0.540B | 0.668B | 0.0005 |
| C18:3n6 | 0.118B | 0.176A | 0.195A | 0.0026 |
| C18:3n3 | 0.5517C | 1.926A | 1.506B | <0.001 |
| C20:3n6 | 1.341A | 0.691B | 1.030AB | 0.0062 |
| C20:3n3 | 0.158A | 0.065B | 0.086B | 0.0013 |
| C20:4n6 | 9.328A | 2.960B | 4.545B | <0.001 |
| C22:4n6 | 3.048A | 0.991B | 1.386B | <0.001 |
| C20:5n3 | 0.430A | 0.125B | 0.201B | <0.001 |
| C22:5n3 | 1.905A | 0.556B | 0.823B | 0.0002 |
| C22:6n3 | 1.016A | 0.316B | 0.481B | <0.001 |
| Total PUFA | 38.70A | 36.115B | 36.356B | 0.0399 |
Abbreviations: MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; SFA, saturated fatty acid.
Pectoralis major muscle associated with Pectoralis minor muscle with DPM score 0: unaffected, score 2: muscles with coagulative necrosis, fibrous tissue texture and necrotic area and score 3: with the damaged muscle tissue having a greenish appearance.
Means followed by distinct letters (in the columns) differ by Tukey's test (P < 0.05).
The polyunsaturated fatty acid (PUFA) are considered to be important nutrients and structural components in cell membranes to regulate human health, utilizing diet as a way of controlling the risk of disease in humans is of considerable interest (Zárate et al., 2017) and higher values in PUFA (P < 0.05) were found in unaffected meats by myopathy, score 0, and lower values from breast fillets with score 2 and 3 (score 0>score 2 = score 3).
However, the C18:1n9c and C18:2n6c are considered to be important for human health too, are higher in meat (P < 0.05) from broilers with myopathy (score 2 = score 3) when compared breast fillet without myopathy (score 0). There were differences in the C14:0 and C16:0 (P < 0.05); meat from breast fillets from broilers with DPM showed higher values than broilers unaffected by DPM.
Cauble et al. (2020), in study of WB affected muscles exhibited a significant higher incidence of white striping found results similar to this DPM myopathy study for some fatty acid profile. The authors conclusion the WB myopathy seemed to be associated with an imbalance of fatty acid profile under analysis as liquid chromatography analysis reveals an imbalance of fatty acid profile in the breast of WB-affected birds with a significant higher percent of saturated fatty acids – SFA (14:0, 15:0, and 17:0) and monounsaturated fatty acids -MUFLA (14:1, 16:1c, 17:1t, 18:1c9, and 18:1c11), and lower content of PUFA and omega-3. The same was observed, highest values (P < 0.05) of breast fillets affected by DPM (score 2 and 3) to SFA (14:0), MUFLA (14:1 and 16:1), and PUFA (18:2n6c).
Differences were detected (P < 0.05) for the pH, WHC, SF, and sarcomere length of the breast fillets (Pectoralis major muscle) samples from broilers affected by DPM (Table 3). The pH values of the breast fillets samples classified as DPM score 2 were higher than meat with absence of the myopathy (score 0) or those with DPM (score 3), and no difference was detected (P > 0.05) between the last 2. According Yalcin et al. (2018) glycogen was replenished and pH of breast fillets muscles with DPM, score 3, was similar to unaffected muscle (score 0) and this studied the occurrence of DPM in broilers and associated alterations in the breast fillets of Ross 308 chickens and found the same trend. The higher pH (P < 0.05) observed in breast fillets samples from birds classified as DPM score 2 (acute lesion) indicates less available glycogen postmortem due to depletion of glycogen stores.
Table 3.
Means and standard deviations of pH, water holding capacity (WHC), cooking loss (CL), shear force (SF) and sarcomere length (SL) of Pectoralis major muscle samples obtained from broilers affected by deep pectoral myopathy (DPM).
| DPM Score |
||||
|---|---|---|---|---|
| 0 | 2 | 3 | P-value | |
| pH | 6.03 ± 0.19B | 6.17 ± 0.22A | 6.07 ± 0.06B | 0.0042 |
| WHC (%) | 67.40 ± 4.02B | 70.34 ± 2.53AB | 71.01 ± 0.99A | 0.0177 |
| CL (%) | 30.14 ± 1.40A | 29.34 ± 1.39A | 28.75 ± 1.98A | 0.1722 |
| SF (N) | 11.469 ± 0.90B | 12.949 ± 1.26AB | 13.493 ± 1.01A | 0.0108 |
| SL (μm) | 1.62 ± 0.05B | 1.76 ± 0.06A | 1.72 ± 0.07A | <0.0001 |
Pectoralis major muscle associated with Pectoralis minor muscle with DPM score 0: unaffected, score 2: muscles with coagulative necrosis, fibrous tissue texture and necrotic area and score 3: with the damaged muscle tissue having a greenish appearance.
Means followed by distinct letters (in the columns) differ by Tukey's test (P < 0.05).
Higher WHC values were observed in the breast fillets affected by DPM (P < 0.05). Higher WHC values mean reduced exudate losses. The samples classified as DPM score 3 showed the highest WHC and protein content values. The fact that the protein to be maintained intact and more soluble improved protein functionality e high WHC values allow resulting in meat products of better quality.
No difference was observed (P > 0.05) for CL and there were differences for SF (P < 0.05) among samples, with the most breast fillets samples (lower SF values) being those unaffected by the condition DPM. This is possibly because the samples classified as DPM score 3 showed higher (P < 0.05) percentages of insoluble collagen than those harvested from the unaffected birds (Table 4). Insoluble collagen is the most stable form of this protein, meaning it is not reducible, having bonds that are more resistant to heat. Though present in a small proportion of the total protein content, it is responsible for several changes in meat texture during cooking.
Table 4.
Means and standard deviations of collagen percentages of Pectoralis major muscle samples obtained from broilers affected by deep pectoral myopathy (DPM).
| DPM Score |
||||
|---|---|---|---|---|
| Collage (%) | 0 | 2 | 3 | P-value |
| Total | 0.667 ± 0.33A | 0.581 ± 0.17A | 0.648 ± 0.29A | 0.7724 |
| Soluble | 0.570 ± 0.31A | 0.431 ± 0.14A | 0.570 ± 0.19A | 0.3975 |
| Insoluble | 0.131 ± 0.03B | 0.147 ± 0.04AB | 0.265 ± 0.24A | 0.0374 |
Pectoralis major muscle associated with Pectoralis minor muscle with DPM score 0: unaffected, score 2: muscles with coagulative necrosis, fibrous tissue texture and necrotic area and score 3: with the damaged muscle tissue having a greenish appearance.
Means followed by distinct letters (in the columns) differ by Tukey test (P < 0.05).
Sarcomere length was loss (P < 0.05) in the unaffected (score 0) those affected by different degrees of DPM (score 2 and 3), although SF is reversed. Sarcomeres shorter than 2.0 μm are related to tenderness variables (proteolysis and collagen). The higher sarcomere length values observed in the breast fillets samples affected by the myopathy (score 2 and 3) are explained by the principle of sarcomere shortening, which can lead to both hardening or tendering of meat, depending on the degree of shortening. Tijare et al. (2016) in study breast fillets with WS and WB muscle myopathies, just like here for DPM, found sarcomere lengths of muscle samples from each category were assessed, and, interestingly, the sarcomeres of both and WB fillets tended to be longer than normal fillets. Therefore, further research is important to investigate this relationship.
The degrees of DPM did not change the concentrations of soluble and total collagen in the breast fillets of the broilers evaluated in this study. Wight and Siller (1980) reported that in addition to a significant change in color (from pink to green in the tender), the meat became tougher and more fibrous towards the later stages of DPM and this may have happened on the fillet as well.
There was no difference (P > 0.05) for lightness (L*) on the ventral side surface of the breast fillets samples. However, for the dorsal side surface, where the Pectoralis major muscle is in contact with the Pectoralis minor muscle, higher L* values (P < 0.05) were found in the meat from birds affected by the myopathy (Table 5).
Table 5.
Mean values and standard deviation of lightness (L*), red intensity (a*) and yellow intensity (b*) of Pectoralis major muscle samples affected by deep pectoral myopathy (DPM).
| DPM Score |
||||
|---|---|---|---|---|
| 0 | 2 | 3 | P-value | |
| Ventral surface color | ||||
| L | 53.44 ± 3.51A | 52.22 ± 2.24A | 54.02 ± 0.93A | 0.2666 |
| a* | 0.59 ± 0.25B | 1.30 ± 0.78A | 1.05 ± 0.71AB | 0.0196 |
| b* | 11.14 ± 0.25A | 7.77 ± 0.78B | 7.63 ± 0.71B | 0.0007 |
| Dorsal surface color | ||||
| L | 56.49 ± 2.20B | 60.33 ± 2.42A | 61.68 ± 1.32A | <0.0001 |
| a* | 1.42 ± 2.64AB | 2.02 ± 2.09A | 0.95 ± 1.69B | 0.0321 |
| b* | 13.41 ± 1.10A | 10.48 ± 0.94B | 11.12 ± 0.30B | 0.0010 |
Pectoralis major muscle associated with Pectoralis minor muscle with DPM score 0: unaffected, score 2: muscles with coagulative necrosis, fibrous tissue texture and necrotic area and score 3: with the damaged muscle tissue having a greenish appearance.
Means followed by distinct letters (in the columns) differ by Tukey's test (P < 0.05).
The higher a* values detected in the breast fillets samples classified as DPM score 2 were expected, since this category describes the stage in which the lesion in the dorsal side fillet is well-defined and sometimes surrounded by a clear hemorrhagic ring. Kijowski and Konstanczak (2009) investigated DPM in broilers and also observed higher a* values in the breast fillets samples of birds affected by DPM score 2.
In conclusion, although deep pectoral myopathy affects the Pectoralis minor (tender) muscle of broilers, it can also alter the chemical composition (moisture, protein and lipid) and qualitative characteristics, especially the texture and color of the breast fillets or Pectoralis major muscle. This may have a negative economic impact on the meat industry, mainly because the consumer first evaluates the color and therefore the texture of the meat. Therefore, further research is warranted to look into the utilization of meat from affected birds upon processing with production elaboration. DPM in the tender also influences the composition of fatty acid profile of the breast fillets of broilers. This is a disadvantage as it results in decreased nutritional benefits to consumers.
Acknowledgments
ACKNOWLEDGMENTS
The authors thank the São Paulo Research Foundation (FAPESP) for the support provided (case no. 2018/06900-7 and 2019/09707-6).
DISCLOSURES
The authors have no conflicts of interest to report
REFERENCES
- AOAC . 18th ed. Association of Analytical Chemists; Washington, DC: 2005. Official Methods of Analysis. [Google Scholar]
- Bilgili S.F., Hess J. Miopatia peitoral profunda. Informativo traduzido do original Ross Tech 08/48. Aviagen Bras. Tecnol. Campinas. 2008;1:3. [Google Scholar]
- Bligh G.E., Dyer J.W. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Brasil. 1997. Ministério da Agricultura, Pecuária e do Abastecimento. Departamento de Inspeção de Produtos de Origem Animal – DIPOA. Divisão de Normas Técnicas – DN . Decreto Lei no 30. 691, de 2 de março de 1952. Alterado pelo decreto no 1.255 de 25 de junho de 1997. Regulamento da Inspeção Industrial e Sanitária de Produtos de Origem Animal (RIISPOA). Publicado no Diário Oficial da união de 07/07/1952, Seção 1, p.10785. Accessed July 2021. www.planalto.gov.br.
- Cavitt L.C., Youm G.W., Meullenet J.F., Owens C.M., Xiong R. Prediction of poultry meat tenderness using razor blade shear, Allo Kramer shear, and sarcomere length. J. Food Sci. 2004;69:SNQ11–SNQ15. [Google Scholar]
- Cauble R.N., Greene E.S., Orlowski S., Walk C., Bedford M., Apple J., Kidd M.T., Dridi S. Dietary phytase reduces broiler woody breast severity via potential modulation of breast muscle fatty acid profiles. Poult. Sci. 2020;99:4009–4015. doi: 10.1016/j.psj.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross H.R., Carpenter Z.L., Smith G.C. Effects of intramuscular collagen and elastin on bovine muscle tenderness. J. Food Sci. 1973;38:998–1003. [Google Scholar]
- Cross H.R., West R.L., Dutson T.R. Comparison of methods for measuring sarcomere length in beef semitendinosus muscle. Meat Sci. 1981;5:261–266. doi: 10.1016/0309-1740(81)90016-4. [DOI] [PubMed] [Google Scholar]
- Daughtry M.R., Berio E., Shen Z, Shah N., Geiger A.E., Berguson E.R., Dalloul R.A., Persia M.E., Shi H., Gerrard D.E. Satellite cell-mediated breast muscle regeneration decreases with broiler size. Poult. Sci. 2017;96:3457–3464. doi: 10.3382/ps/pex068. [DOI] [PubMed] [Google Scholar]
- Hadlich J.C., Morales D.C., Silveira A.C., Oliveira H.N., Chardulo L.A.L. Efeito do colágeno na maciez da carne de bovinos de distintos grupos genéticos. Anim. Sci. 2006;28:57–62. [Google Scholar]
- Hamm R. Biochemistry of meat hydration. Adv. Food. Res. 1961;10:355–463. doi: 10.1016/s0065-2628(08)60141-x. [DOI] [PubMed] [Google Scholar]
- Honikel K.O. The water binding of meat. Fleischwirttsch. 1987;67:1098–1102. [Google Scholar]
- Lee Y.S., Owens C.M., Meullenet J.F. The Meullenet-Owens Razor Shear (MORS) for prediction poultry meat tenderness: its applications and optimization. J. Texture Stud. 2008;39:655–672. [Google Scholar]
- Maia E.L., Rodriguez-Amaya D.B. Avaliação de um método simples e econômico para metilação de ácidos graxos de lipídeos de diversas espécies de peixes. Rev. Inst. Adolfo Lutz. 1993;53:27–35. [Google Scholar]
- Mazzoni M., Petracci M., Meluzzi A., Cavani C., Clavenzani P., Sirri F. Relationship between pectoralis major muscle histology and quality traits of chicken meat. Poult. Sci. 2015;94:123–130. doi: 10.3382/ps/peu043. [DOI] [PubMed] [Google Scholar]
- Petracci M, Cavani C. Muscle growth and poultry meat quality issues. Nutrients. 2012;4:1–12. doi: 10.3390/nu4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petracci M., Soglia F., Zambonelli P., Davoli R., Cavani C. Broiler Pectoralis Major muscle affected by emerging abnormalities: composition and technological traits. Ital. J. Anim. Sci. 2015;14:59. [Google Scholar]
- Kijowski J., Konstanczak M. Deep pectoral myopathy in broiler chickens. Bull. Vet. Inst. Pulawy. 2009;53:487–491. [Google Scholar]
- Kijowski J, Kupińska E. The evaluation of selected quality parameters of broiler chicken muscles with Deep Pectoral Myopathy (DPM) symptoms. J. Eur. Poult. Sci. Arch. Geflügelk. 2013;77:102–108. [Google Scholar]
- Kuttappan V.A., Brewer V.B., Apple J.K., Waldroup P.W., Owens C.M. Influence of growth rate on the occurrence of white striping in broiler breast fillets. Poult. Sci. 2012;91:2677–2685. doi: 10.3382/ps.2012-02259. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Shivaprasad H.L., Shaw D.P., Valentine B.A., Hargis B.M., Clark F.D., Mckee S.R., Owens C.M. Pathological changes associate with white striping in broiler breast muscles. Poult. Sci. 2013;92:331–338. doi: 10.3382/ps.2012-02646. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Hargis B.M., Owens C.M. White striping and woody breast myopathies in the modern poultry industry: a review. Poult. Sci. 2016;95:2724–2733. doi: 10.3382/ps/pew216. [DOI] [PubMed] [Google Scholar]
- Saldanha T, Mazalli M.R., Bragagnolo N. Avaliação comparativa entre dois métodos para determinação do colesterol em carnes e leite. Ciência Tecnol. Alimentos. 2004;24:109–113. [Google Scholar]
- SAS Institute . SAS Institute Inc; Cary, NC: 2002. User's Guide: Statistics. Release 9.1. [Google Scholar]
- Stangierski J., Tomaszewska-Gras J., Baranowska H.M., Krzywdzińska-Bartkowiak M., Konieczny P. The effect of deep pectoral myopathy on the properties of broiler chicken muscles characterised by selected instrumental techniques. Eur. Food Res. Technol. 2019;245:459–467. [Google Scholar]
- Tijare V.V., Yang F.L., Kuttappan V.A., Alvarado C.Z., Coon C.N., Owens C.M. Meat quality of broiler breast fillets with white striping and woody breast muscle myopathies. Poul. Sci. 2016;95:2167–2173. doi: 10.3382/ps/pew129. [DOI] [PubMed] [Google Scholar]
- USDA . United States Department of Agriculture; 2020. Economic Research Service.https://apps.fas.usda.gov/psdonline/circulars/livestock_poultry.pdf Accessed July 2020. [Google Scholar]
- Vieira T.B., Almeida D.O., Alves F.M.X., Franco R.M., Andrade C.L.A., Tortelly R. Aspectos anatomopatológicos da miopatia peitoral profunda em frangos de corte abatidos sob inspeção sanitária. Rev. Bras. Ciência Vet. 2006;13:144–146. [Google Scholar]
- Wight P.A., Siller W.G. Pathology of deep pectoral myopathy of broilers. Vet. Pathol. 1980;17:29–39. doi: 10.1177/030098588001700103. [DOI] [PubMed] [Google Scholar]
- Woessner Junior JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this amino acid. Arch. Biochem. Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- Yalcin T.E., Ilbasmis-Tamer S., Takka S. Development and characterization of gemcitabine hydrochloride loaded lipid polymer hybrid nanoparticles (LPHNs) using central composite design. Int. J. Pharm. 2018;548:255–262. doi: 10.1016/j.ijpharm.2018.06.063. [DOI] [PubMed] [Google Scholar]
- Zárate R., JaberVazdekis N., Tejera N., Pérez J.A., Rodríguez C. Signifcance of long chain polyunsaturated fatty acids in human health. Clin. Trans. Med. 2017;6:25. doi: 10.1186/s40169-017-0153-6. [DOI] [PMC free article] [PubMed] [Google Scholar]