Figure 4. Cerebral organoid RNA sequencing revealed major differentiation defects.
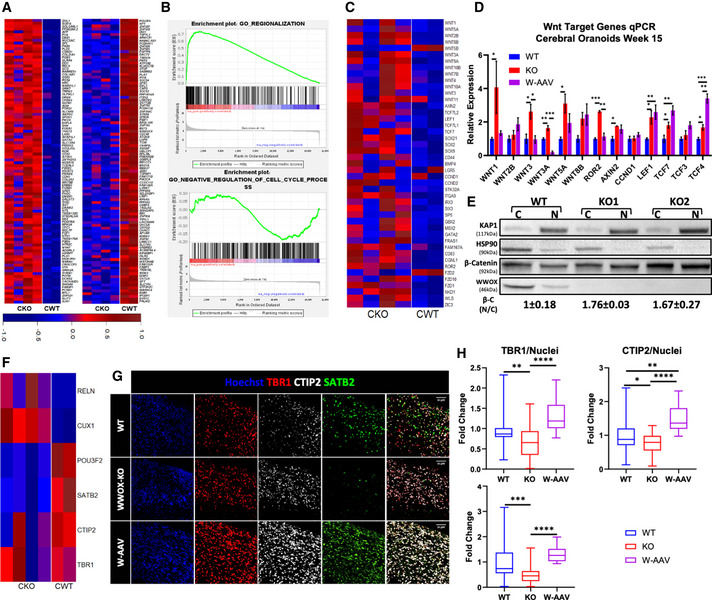
- Heatmap of 100 upregulated genes (left panel) and 100 downregulated genes (right panel) selected by highest fold change.
- Gene set enrichment analysis (GSEA) revealed enrichment of genes related to regionalization of the organoids, and decreased expression of genes related to negative regulation of the cell cycle.
- Heatmap of Wnt pathway‐related genes in 15‐week COs.
- qPCR analysis for selected Wnt target genes validating the results of the RNA‐seq. The y‐axis indicates relative expression fold change. Data are represented as mean ± SEM. Statistical significance was determined using one‐way ANOVA with Tukey’s multiple comparisons test (WT: n = 4 from 1 batch; KO: n = 4 from 1 batch; and W‐AAV: n = 4 from 1 batch).
- Week 16 COs were subfractionated into a cytoplasmic (C) and nuclear (N) fractions. The experiment was run twice with a total of 2 WT organoids and 4 KOs organoids (2 for each KO line). KAP‐1 marks the nucleus, and HSP90 marks the cytoplasm. The numbers at the bottom are a quantification of the nuclear fraction of β‐catenin band intensities normalized to the cytosolic fraction [β‐C (N/C)].
- Heatmap showing the expression levels of markers of the six different layers of the human cortex in week 15 organoids from deepest to the most superficial: TBR1, BCL11B (CTIP2), SATB2, POU3F2 (BRN2), CUX1, and RELN.
- IF staining in week 15 COs validating the decreased levels of the deep layer cortical markers CTIP2 (BCL11B) and TBR1, and superficial layer marker SATB2 (WT: n = 3; KO: n = 4; and W‐AAV: n = 4). Scale = 50 µm.
- Quantification of the cortical markers seen in G, normalized to the total number of nuclei. The y‐axis indicates the fold change compared with the average of the WT COs. The boxplot represents the 1st and 3rd quartile, with its whiskers showing the minimum and maximum points and a central band representing the median. Statistical significance was determined using one‐way ANOVA with Tukey’s multiple comparisons test (WT: n = 9 from 3 batches; KO: n = 16 from 3 batches; and W‐AAV: n = 4 organoids from 1 batch).
Data information: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001.
