Abstract
A 72-year-old patient was admitted to the intensive care unit due to acute respiratory distress syndrome caused by COVID-19. On day 20, the patient experienced shock. The electrocardiogram showed ST segment elevation in leads V3–V6 and severe left ventricular dysfunction with an ejection fraction of 35%–40%. The left ventricle showed basal hypokinesis and apical akinesis, while the creatine kinase level was normal, indicating Takotsubo cardiomyopathy. On day 24, the patient died of multiple organ failure. In post-mortem biopsy, SARS-CoV-2 antigen was detected in cardiomyocytes by immunostaining. Moreover, SARS-CoV-2 RNA was detected in heart tissue. We need to further analyse the direct link between SARS-CoV-2 and cardiomyocytes.
Keywords: SARS-CoV-2, immunostaining, cardiomyocyte, co-localization
Introduction
Acute cardiac injury (ACI) is a common complication of COVID-19 and is associated with COVID-19 severity and mortality (Li et al., 2020). However, the pathogenesis of ACI remains unknown. Moreover, it is unclear whether ACI observed in patients with COVID-19 directly results from viral infection of the cardiomyocytes, as recent preprint data (Siddiq et al., 2020) indicate that interleukin with SARS-CoV-2 infection can directly affect cardiomyocytes in COVID-19 patients; however, definitive evidence of the presence of the virus in cardiomyocytes is lacking in patients with COVID-19. Postmortem biopsied samples of a patient with COVID-19 and ACI were investigated, and the genome of SARS-CoV-2 was detected in the cardiac tissue using real-time reverse transcription-polymerase chain reaction (RT-PCR). In addition, SARS-CoV-2 antigens and genome were detected in cardiomyocytes using immunohistochemistry and in situ hybridization, respectively.
Case presentation
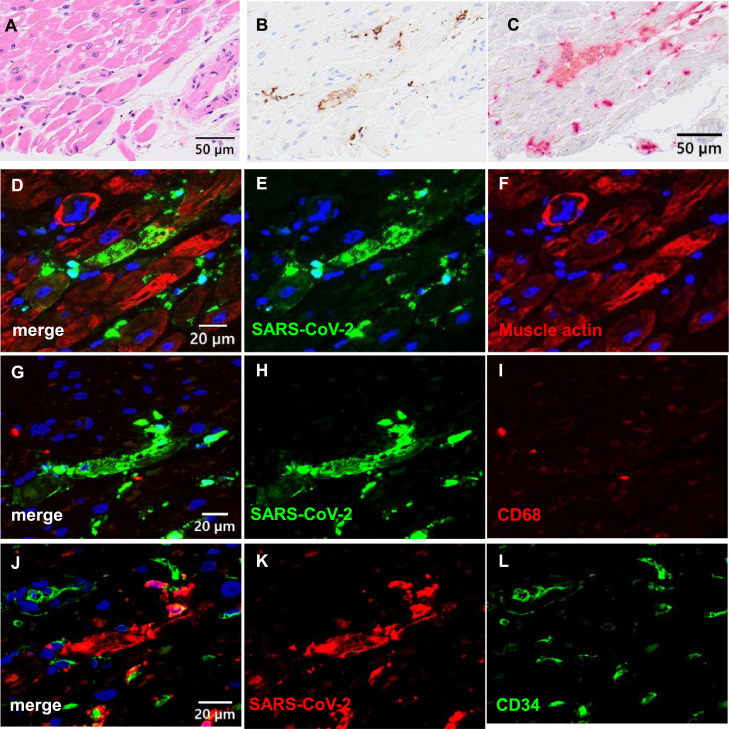
A 72-year-old man was receiving treatment for follicular lymphoma with steroids and chemotherapy in another hospital. His cardiac function was normal, and his ventricular ejection fraction (EF) was 75.6%. During hospitalization, he was infected with SARS-CoV-2 from a patient in another ward. On the day after fever onset, a nasopharyngeal swab sample was obtained and tested using RT-PCR. The RT-PCR was positive for SARS-CoV-2 RNA, and a chest computed tomography scan showed ground-glass opacity in the lung. Therefore, he was diagnosed with COVID-19 pneumonia and treated with dexamethasone and remdesivir. On day 24 after onset, acute respiratory distress syndrome was confirmed, and he was intubated. On day 25 after onset, the patient was transferred to our intensive care unit (ICU). On day 1 in the ICU, an electrocardiogram showed sinus bradycardia (Figure S1-A); the laboratory tests are shown in Table S1. His troponin I level was high, but other laboratory findings did not show evidence of myocardial damage. He was treated with dexamethasone, remdesivir, blood purification, prone therapy, and mechanical ventilation following a lung-protective strategy. On day 20, an electrocardiogram showed V3–V6 ST elevation (Figure S1-B) and apical akinetic expansion with hypokinesia of the mid-ventricular segment, which brought his EF to 35%–40%. However, blood tests showed no creatine kinase elevation, and the troponin I level was slightly increased (Table S1). Takotsubo cardiomyopathy (TTC) was suspected based on these results, and catecholamine support was administered to stabilize his haemodynamics. SARS-CoV-2 RT-PCR tests of the nasopharynx remained positive during his ICU stay, and the patient died of multiple organ failure (MOF) on day 24. A postmortem biopsy was performed on the lung, liver, heart, kidney, and sigmoid colon 1 hour after his death. Histologically, lung sections showed the organizing phase of diffuse alveolar damage with alveolar septal fibrosis and type II pneumocyte hyperplasia (Figure S2). Inflammatory cell infiltration and oedema were observed in the interstitium, and thrombi were observed in the pulmonary arteries. A double immunofluorescence assay (Adachi et al., 2020) detected SARS-CoV-2 antigens in anti-epithelial membrane antigen-positive alveolar or bronchiolar epithelial cells. Heart sections showed focal interstitial myxoid changes and mild fibrosis, but no remarkable changes were observed in cardiac muscle cells. However, immunohistochemistry and in situ hybridization revealed SARS-CoV-2-positive signals in the heart tissue (Figure 1 ). Immunofluorescence revealed that the SARS-CoV-2-positive cells were muscle actin-positive, partially (approximately 20% in SARS-CoV-2-positive cells) CD34-positive, indicating SARS-CoV-2 infection, predominantly in the cardiac muscle cells. Immunohistochemistry detected SARS-CoV-2 antigens in the liver, whereas hepatocytes did not show remarkable changes in the haematoxylin and eosin staining (Figure S3). The kidney showed minor glomerular abnormalities with global sclerosis and chronic tubulointerstitial damage. The colon showed focal erosions with mild inflammation. SARS-CoV-2 antigens were not detected in kidney or colon samples. The copy numbers of SARS-CoV-2 RNA and human glyceraldehyde-3-phosphate dehydrogenase mRNA in specimens were determined using RT-PCR, as described previously (Adachi et al., 2020). SARS-CoV-2 RNA was detected in all samples, including the lung, kidney, heart, liver, and serum (Table 1 ). High copy numbers of the virus were detected in lung samples, corresponding to the immunohistochemistry results for SARS-CoV-2 antigens. Whole genome sequencing of SARS-CoV-2 from organs showed that several additional mutations were detected in the virus from the heart samples compared with the samples derived from the lung, liver, and kidney (Table S2).
Figure 1.
Localization of SARS-CoV-2 in heart tissue. (A, B) Haematoxylin and eosin (HE) staining and immunohistochemistry for SARS-CoV-2 in serial sections of the heart. SARS-CoV-2 is detected in myocardial cells. (C) In situ hybridization for SARS-CoV-2. Red signals are positive for SARS-CoV-2. (D-L) Double immunofluorescence assay in the heart. SARS-CoV-2 antigen is detected in HHF35 (muscle actin) antibody (Dako)-positive cells; however, it is rarely observed in CD68 (PGM1, macrophage)-positive cells and CD34 (endothelial cells)-positive cells. The red, green, and blue signals indicate Alexa 568, 468, and DAPI, respectively. SARS-CoV-2 proteins were labelled with Alexa 468 in panels (D-I) and Alexa 568 in panels (J-L).
Table 1.
SARS-CoV-2 and human GAPDH mRNA copy by real-time RT-PCR.
| Sample | SARS-CoV-2 | GAPDH | SARS-CoV-2 per 1 ng RNA |
|---|---|---|---|
| Lung | 226159.7 | 288.5 | 1617.6 |
| Liver | 104362.6 | 265.2 | 186.9 |
| Kidney | 570.5 | 289.5 | 6.5 |
| Heart | 8514.6 | 17275 | 148.4 |
| Colon | 10185.1 | 4629 | 74.1 |
| Serum# | 7654.9* | 12.3* | 3000 |
Sample is corrected to ICU day 16
Copy number per 1 µL serum
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RT-PCR, reverse transcription polymerase chain reaction; mRNA, messenger ribonucleic acid; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; ICU, intensive care unit.
Discussion
The causes of ACI by SARS-CoV-2 are broadly classified into 2 mechanistic types: direct and indirect. The former refers to the direct myocardial invasion of SARS-CoV-2 into the heart tissue mediated by angiotensin-converting enzyme 2 (ACE2), and the latter is either a cytokine storm or hypoxia-induced cardiac myocyte apoptosis (Clerkin et al., 2020). Histopathological studies have reported organotropism of SARS-CoV-2 beyond the respiratory tract, including renal, myocardial, neurologic, pharyngeal, gastrointestinal and ocular tissues; the pathophysiology underlying cardiovascular manifestations is probably multifactorial (Gupta et al., 2020, Eriksen et al., 2021). ACE2 is highly expressed in cardiovascular tissues, including cardiac myocytes, fibroblasts, endothelial cells, and smooth muscle cells (Gallagher et al., 2008, Hamming et al., 2004). Oudit et al. hypothesized that the interaction between SARS-CoV and ACE2 in the heart contributes to SARS-mediated myocardial inflammation and damage. The authors reported that SARS-CoV-2 RNA was detected in autopsied human heart samples, suggesting direct myocyte invasion of the virus (Oudit et al., 2009). In an in vitro study, SARS-CoV-2 infected cardiomyocytes in an ACE2-and cathepsin-dependent manner (Bojkova et al., 2020). Tavazzi et al. reported the biopsy-proven myocardial localization of viral particles with characteristic morphology and size typical of coronaviruses in a patient with COVID-19 who presented with cardiogenic shock; these particles were observed in structurally damaged interstitial cells but not in cardiomyocytes (Tavazzi et al., 2020). Bojkova et al. detected SARS-CoV-2 particles in cardiomyocytes using heart tissue biopsy collected from a patient with COVID-19 with reduced ventricular EF (Bojkova et al., 2020). This report is the first to provide direct evidence of the localization of viral antigens and genome in cardiomyocytes by immunohistochemistry and in situ hybridization. Additional mutations of SARS-CoV-2 from the heart suggest that virus replication occurs in the heart tissue. These data imply that SARS-CoV-2 may directly injure cardiac tissue of patients with immunodeficiency.
In the present case, the cause of heart failure was not clear, but TTC was suspected based on echocardiogram and blood test findings. The TTC mechanism may be associated with a catecholamine surge, resulting in direct and indirect myocardial damage (Pelliccia et al., 2017), but there is no specific histopathological pattern in TTC (Castillo Rivera et al., 2011). The exact mechanism of TTC in COVID-19 remains to be explained (Singh et al., 2020), but the proportion of TTC is 4.2% (5 of 117) in patients with COVID-19 clinically diagnosed with TTC through transthoracic echocardiography (Giustino et al., 2020). In our case, SARS-CoV-2 antigens were detected in multiple organs throughout the clinical course of 1 month, suggesting that the patient was in an immunocompromised state. Furthermore, the serum sample contained a high amount of the SARS-CoV-2 genome, which may be because the lung endothelium was damaged by COVID-19 pneumonia, and viremia occurred. The pathology of the SARS-CoV-2 infection in this patient indicates that the ACI mechanism may be both direct and indirect.
Acknowledgments
Authors’ contribution
YN, YI, SM, JM, MK, TK, and HI: treatment, care of the patients, and sample collection. YN, HK, and HI drafted the manuscript. HK, NN, YS, TS, and MK carried out pathological and virus genome analysis. All authors critically revised the report, commented on drafts of the manuscript, and approved the final report.
Acknowledgements
The authors thank Dr Kazuki Nabeshima, Department of Pathology, Fukuoka University for his pathological analysis, Drs Kentaro Itokawa, Rina Tanaka, and Masanori Hashino, Pathogen Genomics Center, National Institute of Infectious Diseases for virus genome analysis. We would like to thank Editage (www.editage.com) for English language editing.
Ethical Approval
Informed consent was obtained from the patient's family for publication of this case report.
Declaration of interests
The authors have no conflict of interest to declare.
Funding
This study was supported in part by Grants-in-Aid from the Japan Agency for Medical Research and Development (AMED) (grant numbers; JP20fk0108104 and JP20ek0109476).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2021.08.015.
Appendix. Supplementary materials
References
- Adachi T, Chong JM, Nakajima N, Sano M, Yamazaki J, Miyamoto I, et al. Clinicopathologic and Immunohistochemical Findings from Autopsy of Patient with COVID-19. Japan. Emerg Infect Dis. 2020;26:2157–2161. doi: 10.3201/eid2609.201353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojkova D, Wagner JUG, Shumliakivska M, Aslan GS, Saleem U, Hansen A, et al. SARS-CoV-2 infects and induces cytotoxic effects in human cardiomyocytes. Cardiovas Res. 2020;116:2207–2215. doi: 10.1093/cvr/cvaa267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo Rivera AM, Ruiz-Bailén M, Rucabado Aguilar L. Takotsubo cardiomyopathy–a clinical review. Med Sci Monit. 2011;17:Ra135–Ra147. doi: 10.3109/17482941.2013.869346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, et al. COVID-19 and Cardiovascular Disease. Circ. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- Eriksen AZ, Møller R, Makovoz B, Uhl SA, tenOever BR, Blenkinsop TA. SARS-CoV-2 infects human adult donor eyes and hESC-derived ocular epithelium. Cell stem cell. 2021;28 doi: 10.1016/j.stem.2021.04.028. 1205-20.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher PE, Ferrario CM, Tallant EA. Regulation of ACE2 in cardiac myocytes and fibroblasts. Am J Physiol Heart Circ Physiol. 2008;295:H2373–H2379. doi: 10.1152/ajpheart.00426.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustino G, Croft LB, Oates CP, Rahman K, Lerakis S, Reddy VY, et al. Takotsubo Cardiomyopathy in COVID-19. J Am Coll Cardiol. 2020;76:628–629. doi: 10.1016/j.jacc.2020.05.068. https://www.jacc.org/doi/abs/10.1016/j.jacc.2020.05.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JW, Han TW, Woodward M, Anderson CS, Zhou H, Chen YD, et al. The impact of 2019 novel coronavirus on heart injury: A Systematic review and Meta-analysis. Prog Cardiovasc Dis. 2020;63:518–524. doi: 10.1016/j.pcad.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudit GY, Kassiri Z, Jiang C, Liu PP, Poutanen SM, Penninger JM, et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelliccia F, Kaski JC, Crea F, Camici PG. Pathophysiology of Takotsubo Syndrome. Circ. 2017;135:2426–2441. doi: 10.1161/CIRCULATIONAHA.116.027121. [DOI] [PubMed] [Google Scholar]
- Siddiq MM, Chan AT, Miorin L, Yadaw AS, Beaumont KG, Kehrer T, et al. Physiology of cardiomyocyte injury in COVID-19. medRxiv. 2020 doi: 10.1101/2020.11.10.20229294. [DOI] [Google Scholar]
- Singh S, Desai R, Gandhi Z, Fong HK, Doreswamy S, Desai V, et al. Takotsubo Syndrome in Patients with COVID-19: a Systematic Review of Published Cases. SN Compr Clin Med. 2020:1–7. doi: 10.1007/s42399-020-00557-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.