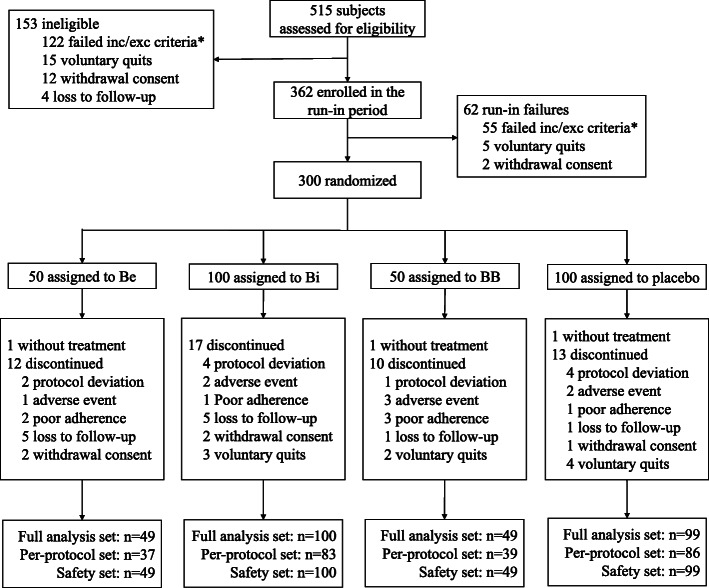
Fig. 1.
Trial profile. *The criteria were not mutually exclusive. The full analysis set, as the primary analysis set for this study, included participants who received at least one dose of the trial drug and had at least one post-treatment data point. The per-protocol set included participants who adhered adequately to the assigned regimen, including undergoing the trial drug treatment according to the protocol without any significant protocol deviation and completing all the evaluations of this study. The safety set included participants who received at least one dose of the trial drug and had at least one safety assessment