Fig. 2.
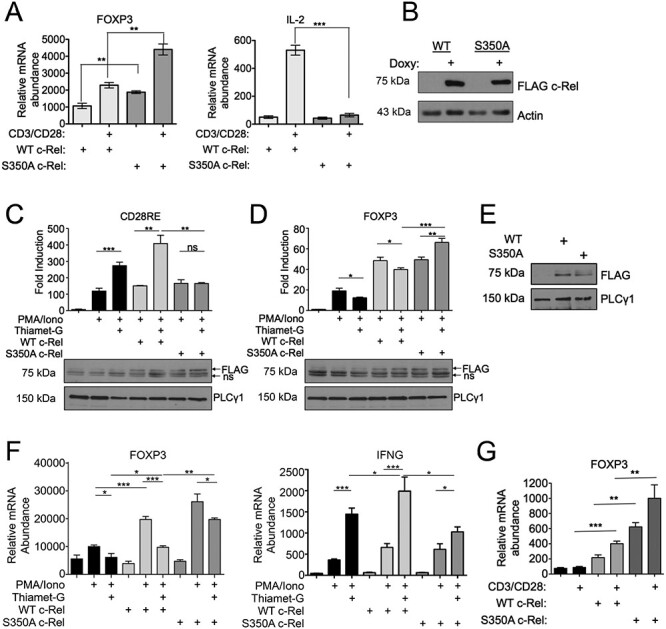
c-Rel S350 O-GlcNAcylation reduces FOXP3 expression, (A) Jurkat T-REx cells (1 × 106) were treated with 1 μg/mL doxycycline for 20 h to induce the expression of FLAG-tagged wild-type or S350A c-Rel. Cells were stimulated in 6-well plates coated with 2 μg/mL anti-CD3 and anti-CD28 antibodies for 3 h. Samples were then analyzed by qPCR to determine the abundance of indicated mRNAs relative to that of TFR. (B) Jurkat T-REx cells were treated with doxycycline as in (A) and FLAG-tagged wild-type or S350A c-Rel expression were examined by western blotting. (C and D) MT-2 cells (5 × 106) were electroporated with CD28RE (C) or FOXP3 CNS3 promoter (D) containing luciferase reporter plasmids and FLAG-tagged wild-type or S350A c-Rel as indicated. Twenty-four hours following transfection, cells were stimulated as indicated with 50 ng/mL PMA, 250 ng/mL Ionomycin and 50 μM Thiamet-G for an additional 24 h. Luciferase activity was assessed using dual luciferase assay system and values are presented as fold induction over luciferase vector alone-transfected sample. Data are representative of three independent experiments each done in triplicates and are presented as mean ± SEM. P values were obtained by unpaired student t-test. Ns—nonsignificant. Data in bar graphs are technical triplicates representative of three independent experiments. Bottom panels show western blotting of luciferase lysates probed for FLAG c-Rel expression. Ns—non specific. (E and F) MT-2 cells (5 × 106) were electroporated with plasmids containing empty vector, FLAG-tagged Wildtype c-Rel or FLAG-tagged S350A c-Rel. (E) Representative blot showing expression of FLAG-tagged wild-type or S350A c-Rel in electroporated MT-2 cell lysates. (F) Cells were stimulated 24 h post electroporation, with 50 ng/mL PMA and 250 ng/mL Ionomycin for 18 h. Samples were then analyzed by qPCR to determine the abundance of indicated mRNAs relative to that of Transferrin Receptor (TFR). (G) CD4+ T cells were isolated from c-Rel knockout C57BL/6J mice and 7.0 × 106 cells per condition were nucleofected with plasmids containing either empty vector, wild-type c-Rel, or S350A c-Rel. After 20 h, cells were stimulated with 2 μg/mL each of plate bound anti-CD3 and anti-CD28 antibodies for 6 h. Samples were then analyzed by qPCR to determine the abundance of FOXP3 mRNA relative to that of Transferrin Receptor (TFR). (A, F, G) Data are representative of three independent experiments, each performed in triplicates, presented as mean ± SEM (n = 3). P values were obtained by unpaired student t-test; *** P < 0.001, ** P < 0.01 * P < 0.05.
