Fig. 3.
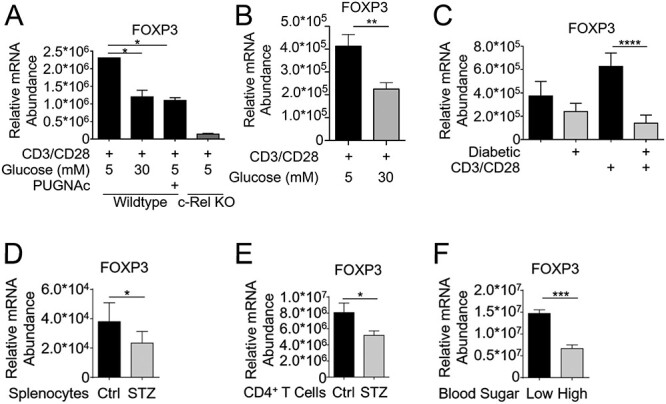
c-Rel O-GlcNAcylation reduces FOXP3 expression in primary T cells. (A) CD4+ T cells (1.5 × 106/condition) isolated from wild-type or c-Rel KO C57BL/6J mice were cultured for 6 h in either low-glucose media (5 mM glucose), low-glucose plus 100 μM PUGNAc or high-glucose (30 mM glucose) conditions. Cells were stimulated in 6-well plates coated with 2 μg/mL anti-CD3 and anti-CD28 antibodies for 6 h. (B) CD4+ T cells from wild-type or c-Rel KO C57BL/6J mice were cultured for 48 h in low or high glucose media and stimulated as in (A). (C) CD4+ T cells were isolated from normal glycemic or hyperglycemic (diabetic) NOD mice, and plated on 6-well plates at 1.5 × 106 cells/well either uncoated or coated with 2 μg/mL anti-CD3 and anti-CD28 antibodies for 6 h. (D) Whole splenocytes were isolated from control or STZ-treated mice and immediately processed for cDNA. (E) CD4+ T cells were isolated from C57BL/6 mice following 9 weeks of Streptozotocin treatment and immediately processed for cDNA. (F) CD4+ T cells were isolated from normal glycemic or hyperglycemic NOD mice, and immediately processed for cDNA. (A–F) Samples were analyzed by qPCR in triplicates to determine the abundance of FOXP3 mRNA relative to that of Ubiquitin Conjugating Enzyme E2 D2 (UBE2D2). Data are presented as mean ± SEM (A–E n = 3, F n = 6). P values were obtained by unpaired student t-test; **** P < 0.0001, *** P < 0.001, ** P < 0.01, * P < 0.05.
