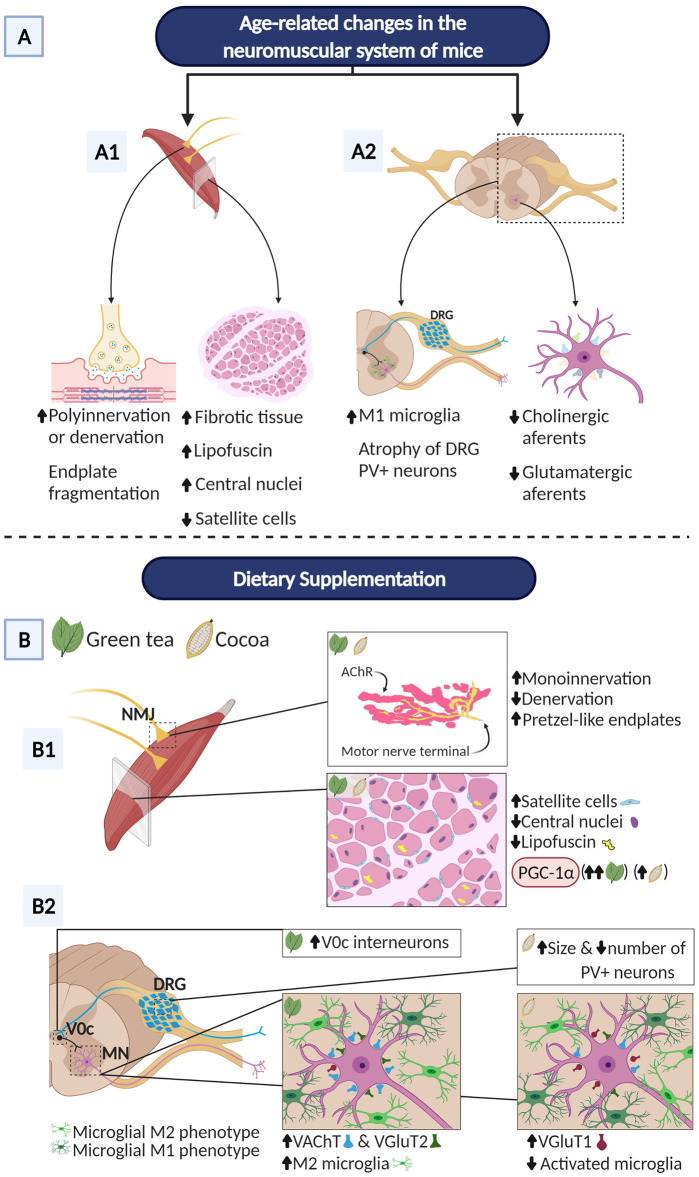
Figure 10.
Overview of main benefits promoted by GTE and cocoa dietary supplementations on aging-associated changes in the neuromuscular system of C57BL/6JRj mice. The hallmark neuromuscular alterations occurring in these mice in the course of aging [6] are also summarized. (A) NMJs of aged mice display signs of either denervation or polyinnervation, and endplate fragmentation, suggesting an active process of NMJ remodeling and muscle reinnervation. Additionally, aged muscles show increased fibrosis, abundant fibers with lipofuscin accumulation and centrally located nuclei (indicative of muscle regeneration), and a marked reduction in the proportion of SCs. Aged mouse spinal cords exhibit reactive gliosis in ventral horn with increased proportion of harmful M1 microglia and significant loss of excitatory cholinergic (C-boutons) and glutamatergic synapses on MNs; atrophy of sensory proprioceptive (PV-positive) DRG neurons was also seen. (B) GTE and cocoa supplementations significantly decrease muscle denervation and signs of NMJ degeneration; both supplements augment the proportion of NMJs exhibiting single innervation, reduce fragmentation of endplates and increase the number of them exhibiting a healthier, “pretzel-like”, appearance. Furthermore, GTE- and cocoa-enriched diets increase the density of satellite cells, and reduce lipofuscin deposition in myofibers and the proportion of them displaying central nuclei. PGC-1α, a key regulatory factor of mitochondrial biogenesis, shows increased muscular levels in animals fed with GTE and cocoa-supplemented diets. GTE-, but not cocoa-, supplementation prevents the aging-associated loss of cholinergic (C-bouton) and VGluT2-positive glutamatergic synapses on lumbar spinal cord MNs; cocoa, but not GTE, increases the density of VGluT1-positive glutamatergic nerve terminals contacting MNs. The prevention of age-related C-bouton loss promoted by GTE is associated with increased numbers of V0C interneurons, the neuronal origin of cholinergic C-bouton inputs to MNs. Additionally, the prevention of aging-associated loss of VGluT1-positive MN-afferents by cocoa is accompanied by the increased body size of PV-positive proprioceptive DRG neurons, the source of Ia VGluT1 afferents to MNs. Moreover, GTE-supplementation improves age-related reactive microgliosis in the spinal cord and increases the proportion of neuroprotective M2 microglial cells around MNs, indicating that the imbalance of M1/M2 microglia found to occur with aging can be potentially modulated by GTE. Created with BioRender.com.