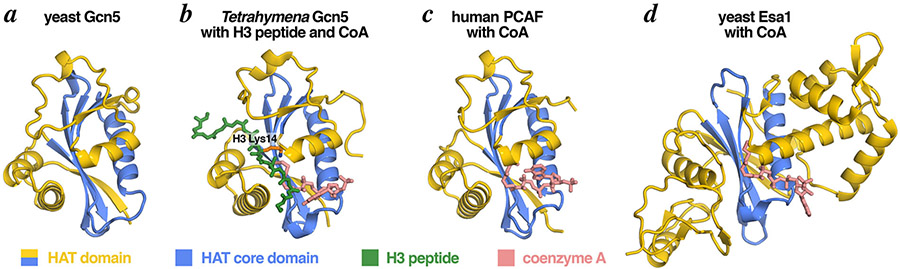
Figure 3: The conserved structure of the Gcn5 HAT domains.
The Gcn5 HAT domain contains a central HAT core domain (blue) and structurally more divergent N- and C-terminal segments (yellow). The HAT core domain is structurally conserved among orthologous Gcn5 enzymes (yeast in panel a, Tetrahymena in panel b), closely related HAT enzymes like PCAF (panel c) and more distantly related HAT enzymes like Esa1 (panel d). The crystal structure of the Tetrahymena ternary complex (panel b) shows Gcn5 bound to its two substrates, CoA (a nonenzymatic surrogate for acetyl-CoA) and an H3 histone peptide. Molecular graphics prepared in PyMOL [66] using PDB coordinates 1YGH, 1PU9, 1CM0, 1MJA.