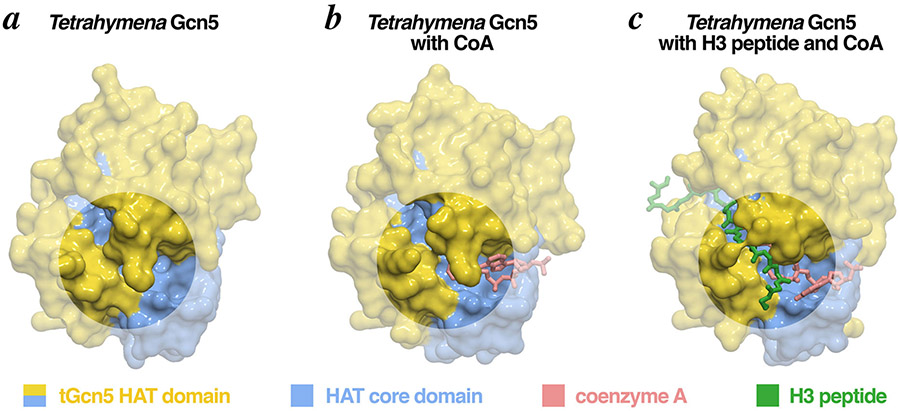
Figure 4: Surface representations of the Gcn5 HAT domain in complex with its substrates.
The Gcn5 HAT domain contains two pronounced clefts which accommodate the histone peptide and acetyl-CoA substrates. Binding of acetyl-CoA to the smaller cleft causes a conformational change that opens the larger, peptide binding cleft, allowing the H3 peptide to bind (circles highlight the conformational changes). Molecular graphics prepared in PyMOL using PDB coordinates 1QST, 1QSR and 1PU9.