Figure 1.
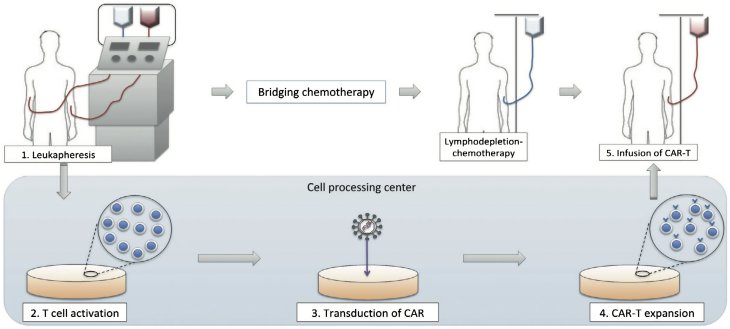
Manufacturing CAR T Cell Therapy
Autologous T cells are collected from the patient. A chimeric receptor antibody (CAR) that recognizes CD19 is inserted into the cell surface using lentiviral or retroviral vectors. The CAR T cells undergo ex vivo rapid multiplication to generate therapeutic quantities. The patient undergoes lymphodepleting chemotherapy (usually a combination of fludarabine and cyclophosphamide) before receiving an infusion of the CAR T cells to reduce the native population of T cells, which in turn promotes CAR T-cell expansion.
Reproduced from Makita et al. (45) with permission.