Abstract
Diagnosis of acute and late cardiotoxicity from cancer therapeutics has become increasingly important as the scope of cardio-oncology increases exponentially, both in terms of the number of people affected and the types of therapies it encompasses. Cardiac magnetic resonance (CMR) is a tool that can offer unparalleled diagnostic information compared with other imaging modalities, but its utilization is often delayed, at the expense of patient care, due to the need for insurance pre-authorization. This paper highlights situations in which CMR is preferred as the diagnostic modality and provides examples of diagnoses more likely to be approved by insurance companies. It also provides specific cardio-oncology diagnoses or questions to help the clinical cardio-oncologist navigate the pre-authorization process.
Key Words: cardiac magnetic resonance, cardio-oncology, cardiotoxicity, chemotherapy, left ventricular dysfunction, pre-authorization
Abbreviations and Acronyms: 2D, 2-dimensional; ACC, American College of Cardiology; CAD, coronary artery disease; CMR, cardiac magnetic resonance; CTRCD, cancer treatment–related cardiac dysfunction; ECV, extracellular volume; GLS, global longitudinal strain; ICI, immune checkpoint inhibitors; LGE, late gadolinium enhancement; LV, left ventricular; LVEF, left ventricular ejection fraction; MACE, major adverse cardiovascular event; RV, right ventricular
Central Illustration
Highlights
-
•
Evolving therapies for cancer improve patient survival but can result in cardiotoxicity.
-
•
CMR can help diagnose, prognosticate, and offer insight to guide the management of cardiotoxicity.
-
•
Pre-authorization for CMR, used by insurance companies, often leads to consequential delays in patient care.
-
•
Advocacy and education of insurance payers and providers are essential to overcome these obstacles.
The specialty of cardio-oncology is a burgeoning field that continues to evolve. Part of this rapid growth is due to advances in oncology, with new treatments that are prolonging survival and increasing cure rates. Many novel therapeutics come with associated cardiovascular effects. In the past, anthracyclines were considered to pose the greatest risk to patients, but this risk was felt to be “manageable” if the dose was kept below a specific level (1). Subsequent experience with anthracyclines has demonstrated considerable cardiotoxicity even at low doses. Newer therapies, although beneficial, have shown myriad cardiac side effects ranging from severe hypertension with tyrosine kinase inhibitors, to life-threatening myocarditis with immune checkpoint inhibitors (ICIs), and severe shock-like dysfunction with chimeric antigen receptor T cell therapy (2). Cancer treatment–related cardiac dysfunction (CTRCD) can occur early due to chemotherapy (3) and may be delayed in the case of radiation therapy, particularly with radiation fields that include incidental dose to cardiac structures or the great vessels (4). In addition, more recent data suggest that there is a high risk of major adverse cardiovascular events (MACE) within the first few years following cardiac radiation exposure (5). The need to diagnose these toxicities rapidly and efficiently is of great concern to both the cardiovascular and oncologic communities. It is estimated that 30% of all patients undergoing cancer therapy will have some cardiovascular issue associated with their care; moreover, cardiovascular disease is the leading cause of morbidity and mortality in the years following cancer treatment (6).
The shifting paradigm of cancer as a chronic disease, with therapy lasting for many years, requires long-term follow-up for ongoing cardiovascular toxicity. As more patients with cancer are treated, achieve remission, and enter survivorship, there is a need to identify those at risk, and to design strategies for how best to monitor long-term cancer therapy–related cardiac disease. In the next 25 years, the number of cancer survivors in the United States is estimated to increase by nearly 11 million: from 15.5 million in 2016 to 26.1 million in 2040. Notably, the proportion of survivors older than age 65 years will increase from 61% to 73%. By 2040, only 18% of cancer survivors will be between the ages of 50 and 64 years, and just 8% will be younger than age 50 years. Thus, the largest proportion of survivors represents an older demographic who may have undergone cardiotoxic therapy and yet are also susceptible to cardiovascular disease due to aging alone or from other comorbid diseases. This is the so called “silver tsunami” population that will undoubtedly require imaging and treatment approaches for cardiovascular complications (7).
CMR imaging is a critical tool for the diagnosis of both early and late cardiotoxic effects (8); however, there are several challenges to having these studies performed in a timely manner, which may impact patient care both in the acute and survivorship phases. In particular, the burden that insurance companies impose by way of pre-authorization has been identified as having an increasingly negative impact on patient care (9). We have worked with the American College of Cardiology (ACC) Cardio-Oncology Section members to identify critical obstacles to the use of CMR in oncology patients. This is expected to affect most patients with cancer at the early phase of their treatment with newer and more potent agents, as well as during surveillance as survival rates continue to increase.
This paper outlines the scope of the problem, the circumstances in which CMR offers the greatest benefit, and arguments for dispensing with onerous pre-authorization which delay care and create a tremendous burden to practicing physicians, especially in the field of cardio-oncology and cancer care. We also outline useful nomenclature and indications when ordering a CMR for a cardio-oncology patient.
Scope of the Pre-authorization Burden
Pre-authorization for testing as a mechanism to contain costs has expanded dramatically of late. Insurance companies frequently create their own guidelines to make coverage determinations, which are often not transparent or publicly available, forcing many practices to repeatedly request testing or medications for their patients, only to be continually denied. In 2020, the American Medical Association conducted a web-based survey of 1,000 practicing physicians who provide patient care more than 20 h per week; 40% were primary care physicians and 60% were specialists. Almost 86% reported that the administrative burden related to prior authorization requests has risen in the past 5 years and 86% of practices reported the pre-authorization burden as “high or extremely high.” On average, a medical practice completes 33 prior authorization requests per physician per week that take 14.4 h to process, with 30% of physicians hiring staff to work exclusively on prior authorizations. Nearly 26% of physicians wait an average of 3 to 5 days, and 7% wait more than 5 days, for authorization (9).
A significant concern for those caring for patients with cancer suspected of having cardiotoxicity is that a delay in treatment is associated with negative outcomes. From the same survey (9):
-
•
24% report that delays from pre-authorization caused serious patient harm;
-
•
16% reported that the delays led to unnecessary hospitalizations;
-
•
91% said pre-authorization caused a delay in access to care;
-
•
74% reported that the delay caused by pre-authorization led the patient to abandon recommended care.
The ACC has also been interested in the burden of pre-authorization. In response to a call to action from ACC Advocacy, members who were practicing cardiologists were asked about the degree that pre-authorization was impacting practice patterns. The data showed several trends (H. McCants, personal communication, May 2019):
-
•
After the initial prior authorization denial, 25% of cases required peer-to-peer consultation;
-
•
42% of denials resulted in the recommendation to perform a different (cheaper) test than the initial request;
-
•
45% of cases required more than 30 min on the phone to resolve, and 30% of cases needed participation from the ordering physician to resolve the authorization;
-
•
60% of submitted cases delayed and/or required rescheduling of care.
Although these reported data were not specific to CMR, CMR was a test that was frequently cited as requiring pre-authorization.
CMR in Anthracycline Chemotherapy
Mitigating cardiotoxicity from cancer therapeutics is the cornerstone of cardio-oncology. Left ventricular ejection fraction (LVEF) is often the parameter of greatest interest when monitoring for cardiotoxicity from oncologic therapies, commonly anthracyclines. CMR is the gold standard noninvasive test to measure LVEF and chamber volumes (10). It has greater spatial resolution than 2-dimensional (2D) echocardiography and radionuclide ventriculography, which are often used to monitor LVEF (11). Geometric assumptions are not required, as in echocardiography, and thus CMR can provide more accurate LVEFs as well as improved right ventricular (RV) and left ventricular (LV) volume quantitation. As a result, CMR can detect subtle changes that predate clinical symptoms from CTRCD. The definition of a significant change in LVEF from cardiotoxic chemotherapeutic agents is a LVEF reduction of >10%, to a value <53% (12). This change can be slight and difficult to detect via 2D echocardiography. A study by Thavendiranathan et al. (13) concluded that there can be a 10% difference in biplane calculation of LVEF from the same echocardiogram when read by 2 different readers. Three-dimensional LVEF by echocardiogram is more accurate and reproducible compared with 2D echocardiography, but this is still not widely available and relies on high-quality images, excellent endocardial detection, and operator expertise. CMR can give more accurate and reproducible results that ultimately guide whether chemotherapy can be safely administered. Expert consensus statements recommend CMR if the LVEF is <53%, if suboptimal image quality precludes accurate measurement of LVEF in those undergoing cardiotoxic chemotherapy, or in those with a late risk of cardiotoxicity (12). This is especially important in those with poor acoustic windows. Decreases in LVEF may also occur due to changes in LV volumes due to dehydration from poor oral intake that can be common with chemotherapy. Meléndez et al. (14) showed that even 3 months after chemotherapy exposure, a decline in LVEF in 20% of patients was due to an isolated decrease in LV end-diastolic volume. This may not be apparent on echocardiography.
Anthracyclines, along with other treatments such as radiation, have clearly identified chronic effects that can result in cardiac damage. Examination of endomyocardial biopsy samples has revealed histologic proof that anthracyclines cause damage to cardiac myocytes (15). Oeffinger et al. (16) noted that long-term survivors of pediatric cancers, many treated with anthracyclines, are more likely to have chronic health conditions that include cardiovascular disease, and to die prematurely compared with untreated controls. The incidence of the most serious outcomes, including cardiovascular disease, increases with time and age (16).
Expert consensus groups, such as American Society of Echocardiography, European Society of Cardiology, and American Society of Clinical Oncology (12,17, 18, 19) recommend serial surveillance via imaging and biomarkers in both symptomatic and asymptomatic patients given the lifelong risk of CTRCD. Imaging and biomarkers should be obtained at baseline; biomarkers should then be obtained with each cycle of cardiotoxic therapy, with imaging performed 6 to 12 months after completion of therapy that includes anthracyclines (12). Repeat imaging should be obtained approximately 5 to 10 years post therapy in asymptomatic patients, and sooner with the onset of any concerning cardiovascular symptoms (12,17).
A small study in adult survivors of pediatric cancer, consisting of patients >10 years from diagnosis who required either anthracyclines and/or radiation, revealed that LVEF was often underestimated by echocardiography, even when 3-dimensional technology was implemented, resulting in a new diagnosis of cardiotoxicity with CMR in more than 30% of patients (20). Asymptomatic RV dysfunction was also noted in another study using CMR to evaluate adult survivors who had previously received anthracyclines (21). Because the RV is often difficult to assess due to its unique shape, CMR remains the modality of choice for RV volumes and function. Another study in patients with previous anthracycline exposure revealed an association between a decline in LV mass and a higher likelihood of adverse cardiovascular events (22) measurable by CMR but not with other imaging modalities. CMR may be able to detect toxic myocardial effects earlier, which may result in modification of treatment to prevent further morbidity.
Strain, or myocardial deformation, has been shown to detect subclinical myocardial dysfunction before an actual drop in LVEF in patients who have previously received anthracyclines (23). A relative reduction in global longitudinal strain (GLS) of more than 15% from the pretreatment baseline is considered as evidence of CTRCD (12). Strain can also be assessed using CMR with algorithms such as displacement encoding with stimulated echoes (DENSE) (24) or fast-SENC by MyoStrain (25). GLS, by both echocardiography and CMR, was found to be a predictor of mortality in patients with ischemic and nonischemic cardiomyopathy (26,27). A small study showed global circumferential and longitudinal strain were lower (less negative or more impaired systolic contraction) in those who had been treated with anthracyclines (28). Thavendiranathan et al. (29) recently showed that cardioprotective therapy based on a decline in GLS resulted in a significantly lower reduction in CTRCD. However, strain can also be pre- and afterload dependent. Jordan et al. (30) observed that 16% of the patients noted in the study by Meléndez et al. (14) had a decrease in circumferential strain due to a decrease in LV end-diastolic volume. Thus, CMR may clarify the etiology of a worsening in strain.
CMR can noninvasively detect diffuse interstitial fibrosis via a technique known as native T1 mapping and calculation of extracellular volume fraction (ECV). Myocardial edema may also be detected and quantified via T2 mapping. Mouse studies have shown that anthracyclines first cause an increase in myocardial edema followed by interstitial fibrosis (31). LV myocardial ECV has been noted to be greater in patients treated with anthracyclines (32, 33, 34), and an elevated native T1 after exposure to cardiotoxic chemotherapy is correlated with a lower LVEF at 18 months. Elevated native T1 was also correlated with a reduction in GLS and a rise in N-terminal proB-type natriuretic peptide (35). This study revealed that abnormalities in native T1 and T2 were able to detect cardiotoxicity better than more traditional parameters such as LVEF and biomarkers (35). Early increases in ECV after exposure to cardiotoxic therapies may reflect acute injury and edema, whereas elevations sometime later are more likely due to fibrosis. Further studies are necessary to assess whether cardioprotective therapy initiated earlier due to abnormal T1, T2, and ECV values leads to improved patient outcomes.
Traditionally, late gadolinium enhancement (LGE) is the most widely used method for myocardial tissue characterization and assessment of focal fibrosis/scar. Animal studies have found that increased LGE after anthracycline exposure was associated with future decline in LVEF as well as evidence of myocyte death on histopathologic examination (36); several human studies have also shown similar findings in the presence of LGE (32,36); however, not all studies have found a similar correlation, and so native T1 and T2 mapping and calculation of ECV need to be incorporated as well.
LV mass is also better characterized by CMR compared with any other noninvasive modality. A decrease in LV mass, presumably due to cardiomyocyte damage with subsequent atrophy, has been found to be associated with MACE in those with anthracycline exposure and offers prognostic information in this population (22). LV mass reductions have also been shown to be correlated with increased heart failure symptoms, even in those with preserved LVEF, suggesting that merely surveilling for LV dysfunction after anthracycline exposure may misclassify some patients who could benefit from cardioprotective therapy, as well as potentially misdiagnose the etiology of their symptoms (37). CMR is also helpful in diagnosing cardiomyopathy due to decreasing myocardial mass and cavity size (Grinch Syndrome) in survivors of pediatric cancer in whom anthracycline exposure at an early age results in heart dimensions that are too small to support the rest of the body and subsequent restrictive physiology (38).
CMR for Assessment of Myocarditis Associated With Immune Checkpoint Inhibitors
ICIs are another class of therapeutic agents that can have potentially fatal cardiotoxic effects, specifically myocarditis. Although uncommon (prevalence 0.6% to 1.3% with single ICI, and 2.4% with 2 ICIs), the possibility of toxicity increases with combination immunotherapy (39,40). This relatively new class of medications helps the immune system to recognize and target cancer cells. In the United States alone, there are nearly 600,000 patients who are eligible to receive ICI therapy and thus potentially at risk for myocarditis (41).
CMR is the imaging modality of choice for patients with acute nonischemic injury and is considered the gold standard noninvasive test for the diagnosis of myocarditis. It allows tissue characterization to identify cardiac inflammation (as seen on T2-weighted images and T2 mapping), as well as myocardial necrosis and fibrosis, as evidenced by LGE. The main strength of CMR when using the Lake Louise criteria is its high specificity (86%) and positive predictive value. The newer modified Lake Louise criteria that use more innovative T1 and T2 mapping techniques, as well as extracellular volume quantification, offer greater diagnostic accuracy for myocarditis (42). As such, it is the most efficient option for confirming suspected myocarditis in patients with a high pretest probability (43).
CMR has also been shown to be effective at-risk stratification and determining prognosis in patients with myocarditis (44,45). Because ICI myocarditis can have a rapidly progressive course, any delay in diagnosis or treatment may be catastrophic. The current standard of care for ICI myocarditis is high-dose steroids, although other immunosuppressive agents such as infliximab (46,47), intravenous immunoglobulin, anti-thymocyte globulin, mycophenolate mofetil, tacrolimus (48), and abatacept (49) have also been used. CMR can provide a rapid diagnosis in this life-threatening and often challenging to diagnose syndrome, and allows other etiologies to be excluded based on tissue characterization. CMR should be performed quickly, and without delays related to pre-authorization, as any postponement in diagnosis can cost patient lives. The National Comprehensive Cancer Network endorses the use of CMR as a diagnostic tool for the evaluation of ICI-related toxicities, including myocarditis, pericarditis, arrhythmias, and impaired ventricular function (50).
The greater utility of CMR in ICI myocarditis relates to its ability to directly assess the state of the myocardium. The diagnosis of ICI myocarditis is extremely challenging because the traditional criteria for diagnosing myocarditis are not always present in this disorder. A normal echocardiogram (specifically a normal LVEF) does not rule out disease, nor does it imply a benign course as compared with the more common viral-related myocarditis. LVEF was normal in 51% of patients with proven ICI myocarditis and 38% of patients who experienced a MACE had a normal LVEF (39). However, a study by Zhang et al. (51) suggested that ICI myocarditis may not result in LGE, nor elevated T2 short tau inversion recovery. A more recent study found that T1 mapping provided diagnostic and prognostic value in patients with ICI myocarditis, when applied via the modified Lake Louise criteria (52). More research is necessary to determine whether other tissue characterization techniques such as T2 mapping and ECV provide additional useful information in the diagnosis of ICI myocarditis. ICI myocarditis can range from subclinical to fulminant, and typically has a high rate of serious adverse cardiovascular events, with grade 4 or 5 cardiovascular adverse events in more than half of cases. Therefore, early recognition, in which CMR can aid, and prompt treatment are imperative.
CMR in Other Cancer Therapies
CMR is useful because it not only provides reproducible and accurate evaluation of biventricular volumes and ejection fraction, but it can also characterize myocardial tissue components and evaluate the pericardium, valves, and great vessels. Pericardial disease can be secondary to cardiac metastasis or a consequence of therapy that includes both radiation and chemotherapy. Agents such as cyclophosphamide, cytarabine, imatinib, dasatinib, interferon-ɑ, methotrexate, and arsenic trioxide, among others, can affect the pericardium (12). Although echocardiography is the initial imaging modality of choice, CMR can be especially helpful in evaluating the location and etiology of both pericardial and cardiac masses, as well as assessment for pericardial thickening, constrictive pericarditis, and active inflammation of the pericardium (53,54). CMR can also diagnose coronary artery disease (CAD) and peripheral artery disease without the use of ionizing radiation, which may be important in patients with cancer who are already exposed to many imaging studies. Radiation is known to accelerate vascular arterial disease, and thus it is recommended that patients with a history of significant radiation exposure are followed for development of complications (17). Both traditional chemotherapy such as 5-fluororacil, and newer agents such as vascular endothelial growth factor inhibitors (ie, sorafenib or sunitinib), can cause ischemia (12). CE-MARC (Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease), which is the largest prospective CMR study, showed that CMR is more sensitive and has a higher negative predictive value for diagnosing CAD than single-photon emission computed tomography (55). Five-year follow-up of the CE-MARC study continued to show that CMR is a stronger predictor of MACE irrespective of cardiovascular risk factors, cardiac angiography results, or patient treatment on presentation (56). CMR can detect as well as quantify calcification without radiation exposure (57), and more recently can quantify and characterize atherosclerotic plaque within the carotid artery (58), although this is still in the research phase at this time.
Cost-Effectiveness of CMR
Factors limiting CMR use have traditionally included cost as well as availability. However, the cost-effectiveness of CMR has not been studied prospectively in comparison with other widely used modalities. The retrospective, multicenter SPINS (Stress CMR Perfusion Imaging in the United States) trial did show that patients without ischemia or scar on CMR experienced very low incidence of cardiac events, nominal need for coronary revascularization, and thus less subsequent testing and treatment for ischemia that resulted in decreased cost in the years to come (59). A small retrospective study of approximately 360 patients from 2 centers showed that using CMR over current standards of care saved more than $800,000 (average savings of $2,308 per patient) due to avoidance of invasive procedures and additional diagnostic testing and had a meaningful clinical impact in nearly 70% of patients (60). Furthermore, these data do not include potential additional cost savings, such as limiting ionizing radiation, which may decrease future risk of cancer. CMR can also take the place of the multiple studies often recommended by insurance companies, who provide pre-authorization without a clinical background. Within the cardio-oncology population, 2 cardiac tests such as stress nuclear testing plus echocardiography are recommended to detect asymptomatic cardiotoxicity in those who have had anthracycline and/or radiation exposure (17, 18, 19). A single stress CMR examination can evaluate for ischemia, RV/LV function, valvular disease, pericardial disease, and possible subclinical myocardial changes, all in one test. There are patient limitations in CMR that need to be considered, such as claustrophobia and risks associated with the presence of ferromagnetic devices. Longer imaging times also may be more difficult for some patients. The newer macrocyclic gadolinium-based contrast agents have decreased the risk of nephrogenic systemic fibrosis in those with renal disease, allowing a greater number of people to undergo CMR with contrast (61).
Navigating CMR Pre-Authorization
Although more research is needed in the cardio-oncology patient population, and advances in CMR continue to expand its usefulness, the preceding data suggest that CMR would offer very relevant information in patients with a prior history of known cardiotoxic treatment, such as anthracyclines or radiation to better assess the ventricles, pericardium, and myocardial tissue. CMR has been shown to be helpful in the diagnosis of ICI myocarditis, and is the gold standard noninvasive imaging modality in diagnosing myocarditis from all causes. It also may provide more relevant information in those who are currently receiving newer, more targeted therapies such as monoclonal antibodies, as knowledge is still evolving regarding the full spectrum of cardiovascular side effects these therapies cause. It has also proven to be more sensitive with a higher negative predictive value in the diagnosis of CAD than single-photon emission computed tomography with the benefit of avoiding additional exposure to ionizing radiation (55) and can offer prognostic information in CAD as well (56). Thus, we advocate that pre-authorization be waived when these are the indications for testing.
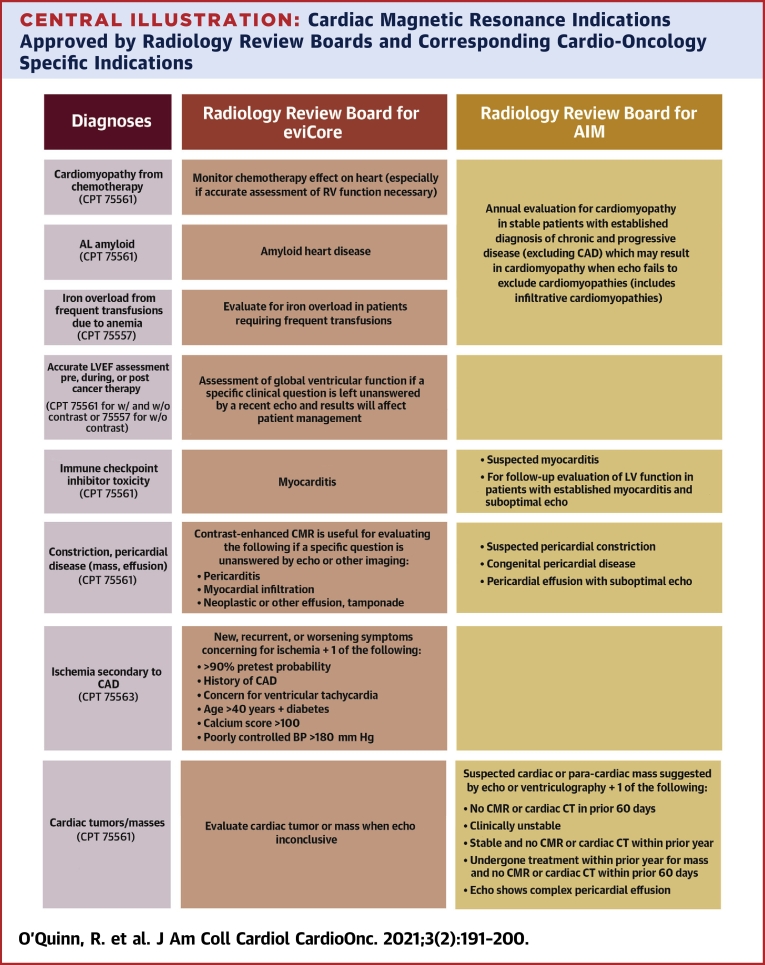
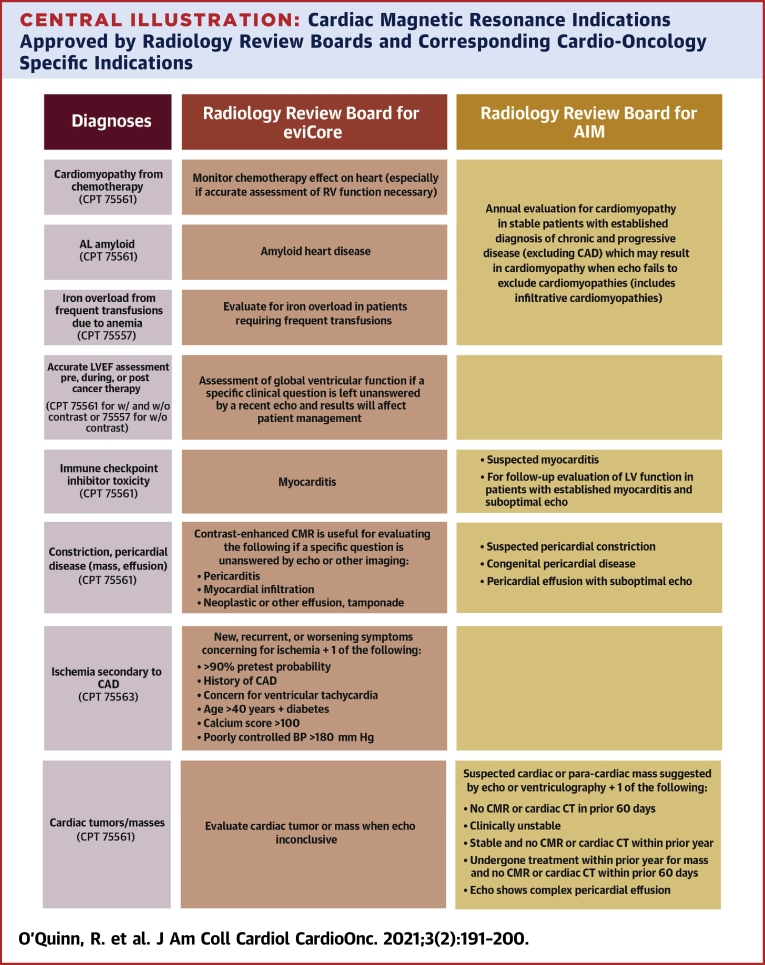
Despite these data supporting instances in which CMR is clearly preferred and indicated in the cardio-oncology population, there is still difficulty with insurers covering CMR examinations, or causing significant delays in authorization (and subsequent care). Many companies use third parties known as radiology review boards to help them screen requests and decide which indications should be covered for various imaging tests. Per these documents, certain diagnoses and diagnosis codes are more likely to be approved. Using the list provided by 2 insurance companies (62,63), the Central Illustration shows their approved indications for CMR, along with the specific cardio-oncology diagnoses or questions to be answered to make it easier for cardio-oncologists who are not as familiar with CMR to convey their reasons for ordering the test and highlighting what indications are often approved by insurance companies.
Central Illustration.
Cardiac Magnetic Resonance Indications Approved by Radiology Review Boards and Corresponding Cardio-Oncology Specific Indications
The approved CMR indications often used by third-party radiology review boards for 2 insurance companies, eviCore and AIM as examples, along with the specific cardio-oncology indications are highlighted. AL = amyloid light-chain; BP = blood pressure; CAD = coronary artery disease; CT = computerized tomography scan; CMR = cardiac magnetic resonance; LV = left ventricular; RV = right ventricular.
Cancer survivors are increasing in number due to advances in cancer therapies. We must be vigilant in both the treatment and survivorship phases to ensure that treating one disease does not lead to another. CMR is one of the best noninvasive diagnostic imaging tools available during cancer treatment, spanning from early diagnosis to accurate late evaluation of cardiac toxicity, as noted previously. Its use is often delayed due to the complexities of health care delivery in the United States. By including the Central Illustration, providers may be able to correctly identify why CMR is necessary but may not eliminate pre-authorization.
The ACC has been active in encouraging prevention of delays due to pre-authorization. Because cardio-oncology is a relatively new field for payers, we hope to continue our efforts to provide education and evidence to ensure the best care for these complex patients. Care teams can help ACC track inappropriate delays in care due to pre-authorization via www.acc.org/partool.
Conclusions
CMR has multiple advantages when evaluating patients for cardiotoxicity with prior exposure to anthracyclines, radiation, monoclonal antibodies, and immunotherapy, in addition to other agents, and thus should be strongly considered in the clinical contexts outlined in this paper. Test stacking using other modalities as required by pre-authorization algorithms leads to increases in costs as well as delays in care that can be life-threatening. We believe that CMR is the most appropriate modality in these instances and should not require a pre-authorization process. We have included a table (Central Illustration) to guide the CMR approval in certain cases. This tool will not eliminate delays in all instances in which CMR is the test of choice, nor does it address all circumstances in which CMR is indicated. Together, we can work to identify inappropriate delays in care due to the pre-authorization process.
Funding Support and Author Disclosures
Dr O’Quinn has received consulting fees for educational endeavors from AstraZeneca and Bracco. Dr Barac has received honoraria for participation in the CV Safety Advisory Board for Takeda Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Jeffrey L. Anderson, MD, served as Guest Associate Editor for this paper. Anju Nohria, MD, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Gilladoga A.C., Manuel C., Tan C.T.C., Wollner N., Sternberg S.S., Murphy M.L. The cardiotoxicity of adriamycin and daunomycin in children. Cancer. 1976;37:1070–1078. doi: 10.1002/1097-0142(197602)37:2+<1070::aid-cncr2820370814>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 2.Zarifa A., Albittar A., Kim P.Y. Cardiac toxicities of anticancer treatments: chemotherapy, targeted therapy and immunotherapy [Editorial] Curr Opin Cardiol. 2019;34:441–450. doi: 10.1097/HCO.0000000000000641. [DOI] [PubMed] [Google Scholar]
- 3.Cardinale D., Colombo A., Bacchiani G. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 4.Donnellan E., Phelan D., McCarthy C.P., Collier P., Desai M., Griffin B. Radiation-induced heart disease: A practical guide to diagnosis and management. Cleve Clin J Med. 2016;83:914–922. doi: 10.3949/ccjm.83a.15104. [DOI] [PubMed] [Google Scholar]
- 5.Atkins K.M., Rawal B., Chaunzwa T.L. Cardiac radiation dose, cardiac disease, and mortality in patients with lung cancer. J Am Coll Cardiol. 2019;73:2976–2987. doi: 10.1016/j.jacc.2019.03.500. [DOI] [PubMed] [Google Scholar]
- 6.Henson K.E., Reulen R.C., Winter D.L. Cardiac mortality among 200 000 five-year survivors of cancer diagnosed at 15 to 39 years of age. Circulation. 2016;134:1519–1531. doi: 10.1161/CIRCULATIONAHA.116.022514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bluethmann S.M., Mariotto A.B., Rowland J.H. Anticipating the “silver tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Prev Biomark. 2016;25:1029–1036. doi: 10.1158/1055-9965.EPI-16-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harries I., Liang K., Williams M. Magnetic resonance imaging to detect cardiovascular effects of cancer therapy. J Am Coll Cardiol CardioOnc. 2020;2:270–292. [Google Scholar]
- 9.Anon. Prior auth survey findings underscore need for legislative action. American Medical Association. https://www.ama-assn.org/practice-management/sustainability/prior-auth-survey-findings-underscore-need-legislative-action Available at:
- 10.Cranney G.B., Lotan C.S., Dean L., Baxley W., Bouchard A., Pohost G.M. Left ventricular volume measurement using cardiac axis nuclear magnetic resonance imaging. Validation by calibrated ventricular angiography. Circulation. 1990;82:154–163. doi: 10.1161/01.cir.82.1.154. [DOI] [PubMed] [Google Scholar]
- 11.Bellenger N. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance. Are they interchangeable? Eur Heart J. 2000;21:1387–1396. doi: 10.1053/euhj.2000.2011. [DOI] [PubMed] [Google Scholar]
- 12.Plana J.C., Galderisi M., Barac A. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–939. doi: 10.1016/j.echo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Thavendiranathan P., Grant A.D., Negishi T., Plana J.C., Popović Z.B., Marwick T.H. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol. 2013;61:77–84. doi: 10.1016/j.jacc.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 14.Meléndez G.C., Sukpraphrute B., D’Agostino R.B. Frequency of left ventricular end-diastolic volume-mediated declines in ejection fraction in patients receiving potentially cardiotoxic cancer treatment. Am J Cardiol. 2017;119:1637–1642. doi: 10.1016/j.amjcard.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ewer M.S., Ali M.K., Mackay B. A comparison of cardiac biopsy grades and ejection fraction estimations in patients receiving Adriamycin. J Clin Oncol. 1984;2:112–117. doi: 10.1200/JCO.1984.2.2.112. [DOI] [PubMed] [Google Scholar]
- 16.Oeffinger K.C., Kawashima T., Friedman D.L., Kadan-Lottick N.S., Robison L.L. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;11:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 17.Lancellotti P., Nkomo V.T., Badano L.P. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2013;14:721–740. doi: 10.1093/ehjci/jet123. [DOI] [PubMed] [Google Scholar]
- 18.Zamorano J.L., Lancellotti P., Rodriguez Muñoz D. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 19.Armenian S.H., Lacchetti C., Barac A. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;35:893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong G.T., Oeffinger K.C., Chen Y. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31:3673–3680. doi: 10.1200/JCO.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ylänen K., Poutanen T., Savikurki-Heikkilä P., Rinta-Kiikka I., Eerola A., Vettenranta K. Cardiac magnetic resonance imaging in the evaluation of the late effects of anthracyclines among long-term survivors of childhood cancer. J Am Coll Cardiol. 2013;61:1539–1547. doi: 10.1016/j.jacc.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 22.Neilan T.G., Coelho-Filho O.R., Pena-Herrera D. Left ventricular mass in patients with a cardiomyopathy after treatment with anthracyclines. Am J Cardiol. 2012;110:1679–1686. doi: 10.1016/j.amjcard.2012.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thavendiranathan P., Poulin F., Lim K.-D., Plana J.C., Woo A., Marwick T.H. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63:2751–2768. doi: 10.1016/j.jacc.2014.01.073. [DOI] [PubMed] [Google Scholar]
- 24.Aletras A.H., Ding S., Balaban R.S., Wen H. DENSE: displacement encoding with stimulated echoes in cardiac functional MRI. J Magn Reson. 1999;137:247–252. doi: 10.1006/jmre.1998.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myocardial Solutions Prefect Study of MyoStrain CMR for the Detection of Cardiotoxicity. clinicaltrials.gov;. 2020. https://clinicaltrials.gov/ct2/show/NCT03543228 Available at:
- 26.Stanton T., Leano R., Marwick T.H. Prediction of all-cause mortality from global longitudinal speckle strain. Circ Cardiovasc Imaging. 2009;2:356–364. doi: 10.1161/CIRCIMAGING.109.862334. [DOI] [PubMed] [Google Scholar]
- 27.Romano S., Judd R.M., Kim R.J. Feature-tracking global longitudinal strain predicts death in a multicenter population of patients with ischemic and nonischemic dilated cardiomyopathy incremental to ejection fraction and late gadolinium enhancement. J Am Coll Cardiol Img. 2018;11:1419–1429. doi: 10.1016/j.jcmg.2017.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lunning M.A., Kutty S., Rome E.T. Cardiac magnetic resonance imaging for the assessment of the myocardium after doxorubicin-based chemotherapy. Am J Clin Oncol. 2015;38:377–381. doi: 10.1097/COC.0b013e31829e19be. [DOI] [PubMed] [Google Scholar]
- 29.Thavendiranathan P., Negishi T., Somerset E. Strain-guided management of potentially cardiotoxic cancer therapy. J Am Coll Cardiol. 2021;77:392–401. doi: 10.1016/j.jacc.2020.11.020. [DOI] [PubMed] [Google Scholar]
- 30.Jordan J.H., Todd R.M., Vasu S., Hundley W.G. Cardiovascular magnetic resonance in the oncology patient. J Am Coll Cardiol Img. 2018;11:1150–1172. doi: 10.1016/j.jcmg.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farhad H., Staziaki P.V., Addison D. Characterization of the changes in cardiac structure and function in mice treated with anthracyclines using serial cardiac magnetic resonance imaging. Circ Cardiovasc Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.115.003584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neilan T.G., Coelho-Filho O.R., Shah R.V. Myocardial extracellular volume by cardiac magnetic resonance imaging in patients treated with anthracycline-based chemotherapy. Am J Cardiol. 2013;111:717–722. doi: 10.1016/j.amjcard.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lustberg M.B., Reinbolt R., Addison D. Early detection of anthracycline-induced cardiotoxicity in breast cancer survivors with T2 cardiac magnetic resonance. Circ Cardiovasc Imaging. 2019;12 doi: 10.1161/CIRCIMAGING.118.008777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jordan J.H., Vasu S., Morgan T.M. Anthracycline-associated T1 mapping characteristics are elevated independent of the presence of cardiovascular comorbidities in cancer survivors. Circ Cardiovasc Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.115.004325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haslbauer J.D., Lindner S., Valbuena-Lopez S. CMR imaging biosignature of cardiac involvement due to cancer-related treatment by T1 and T2 mapping. Int J Cardiol. 2019;275:179–186. doi: 10.1016/j.ijcard.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 36.Lightfoot J.C., D’Agostino R.B., Hamilton C.A. Novel approach to early detection of doxorubicin cardiotoxicity by gadolinium-enhanced cardiovascular magnetic resonance imaging in an experimental model. Circ Cardiovasc Imaging. 2010;3:550–558. doi: 10.1161/CIRCIMAGING.109.918540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jordan J.H., Castellino S.M., Meléndez G.C. Left ventricular mass change after anthracycline chemotherapy. Circ Heart Fail. 2018;11 doi: 10.1161/CIRCHEARTFAILURE.117.004560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lipshultz S.E., Scully R.E., Stevenson K.E. Hearts too small for body size after doxorubicin for childhood ALL: Grinch syndrome. J Clin Oncol. 2014;32:10021. [Google Scholar]
- 39.Mahmood S.S., Fradley M.G., Cohen J.V. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tajiri K., Ieda M. Cardiac complications in immune checkpoint inhibition therapy. Front Cardiovasc Med. 2019;6:3. doi: 10.3389/fcvm.2019.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andrews A. Treating with checkpoint inhibitors—figure $1 million per patient. Am Health Drug Benefits. 2015;8:9. [PMC free article] [PubMed] [Google Scholar]
- 42.Ferreira V.M., Schulz-Menger J., Holmvang G. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 43.Johnson D.B., Balko J.M., Compton M.L. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375:1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gräni C., Eichhorn C., Bière L. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol. 2017;70:1964–1976. doi: 10.1016/j.jacc.2017.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang F., Wang J., Li W. The prognostic value of late gadolinium enhancement in myocarditis and clinically suspected myocarditis: systematic review and meta-analysis. Eur Radiol. 2020;30:2616–2626. doi: 10.1007/s00330-019-06643-5. [DOI] [PubMed] [Google Scholar]
- 46.Padegimas A., Agarwal P., Fleitman J. Case series of ventricular tachycardia and myocarditis from programmed cell-death protein-1 inhibitor treated with infliximab. J Am Coll Cardiol EP. 2019;5:989–992. doi: 10.1016/j.jacep.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Zhang R.S., Padegimas A., Murphy K.M. Treatment of corticosteroid refractory immune checkpoint inhibitor myocarditis with Infliximab: a case series. Cardio-Oncol. 2021;7:13. doi: 10.1186/s40959-021-00095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ganatra S., Neilan T.G. Immune checkpoint inhibitor-associated myocarditis. Oncologist. 2018;23:879–886. doi: 10.1634/theoncologist.2018-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salem J.-E., Allenbach Y., Vozy A. Abatacept for severe immune checkpoint inhibitor-associated myocarditis. N Engl J Med. 2019;380:2377–2379. doi: 10.1056/NEJMc1901677. [DOI] [PubMed] [Google Scholar]
- 50.Thompson J.A., Schneider B.J., Brahmer J. Management of immunotherapy-related toxicities, version 1.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:255–289. doi: 10.6004/jnccn.2019.0013. [DOI] [PubMed] [Google Scholar]
- 51.Zhang L., Awadalla M., Mahmood S.S. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur Heart J. 2020;41:1733–1743. doi: 10.1093/eurheartj/ehaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thavendiranathan P., Zhang L., Zafar A. Myocardial T1 and T2 mapping by magnetic resonance in patients with immune checkpoint inhibitor-associated myocarditis. J Am Coll Cardiol. 2021;77:1503–1516. doi: 10.1016/j.jacc.2021.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aldweib N., Farah V., Biederman R.W.W. Clinical utility of cardiac magnetic resonance imaging in pericardial diseases. Curr Cardiol Rev. 2018;14:200–212. doi: 10.2174/1573403X14666180619104515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klein A.L., Abbara S., Agler D.A. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease. J Am Soc Echocardiogr. 2013;26:965–1012.e15. doi: 10.1016/j.echo.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 55.Greenwood J.P., Maredia N., Younger J.F. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet. 2012;379:453–460. doi: 10.1016/S0140-6736(11)61335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Greenwood J.P., Herzog B.A., Brown J.M. Prognostic value of cardiovascular magnetic resonance and single-photon emission computed tomography in suspected coronary heart disease: long-term follow-up of a prospective, diagnostic accuracy cohort study. Ann Intern Med. 2016;165:1–9. doi: 10.7326/M15-1801. [DOI] [PubMed] [Google Scholar]
- 57.Truijman M.T.B., Kooi M.E., van Dijk A.C. Plaque at RISK (PARISK): prospective multicenter study to improve diagnosis of high-risk carotid plaques. Int J Stroke. 2014;9:747–754. doi: 10.1111/ijs.12167. [DOI] [PubMed] [Google Scholar]
- 58.Golledge J., Siew D.-A. Identifying the carotid ‘high risk’ plaque: is it still a riddle wrapped up in an enigma? Eur J Vasc Endovasc Surg. 2008;35:2–8. doi: 10.1016/j.ejvs.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 59.Kwong R.Y., Ge Y., Steel K. Cardiac magnetic resonance stress perfusion imaging for evaluation of patients with chest pain. J Am Coll Cardiol. 2019;74:1741–1755. doi: 10.1016/j.jacc.2019.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hegde V.A., Biederman R.W., Mikolich J.R. Cardiovascular magnetic resonance imaging—incremental value in a series of 361 patients demonstrating cost savings and clinical benefits: an outcome-based study. Clin Med Insights Cardiol. 2017;11 doi: 10.1177/1179546817710026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schieda N., Blaichman J.I., Costa A.F. Gadolinium-based contrast agents in kidney disease: a comprehensive review and clinical practice guideline issued by the Canadian Association of Radiologists. Can J Kidney Health Dis. 2018;5 doi: 10.1177/2054358118778573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.eviCore Healthcare Cardiac Imaging Guidelines. https://www.evicore.com/-/media/files/evicore/clinical-guidelines/solution/cardiology-and-radiology/2021/evicore_cardiac_final_v10_eff020121_pub11052020_upd12112020.pdf Available at:
- 63.AIM Specialty Health Cardiac Imaging Guidelines. https://aimspecialtyhealth.com/wp-content/uploads/2019/11/AIM_Guidelines_Cardiac_Imaging.pdf Available at.