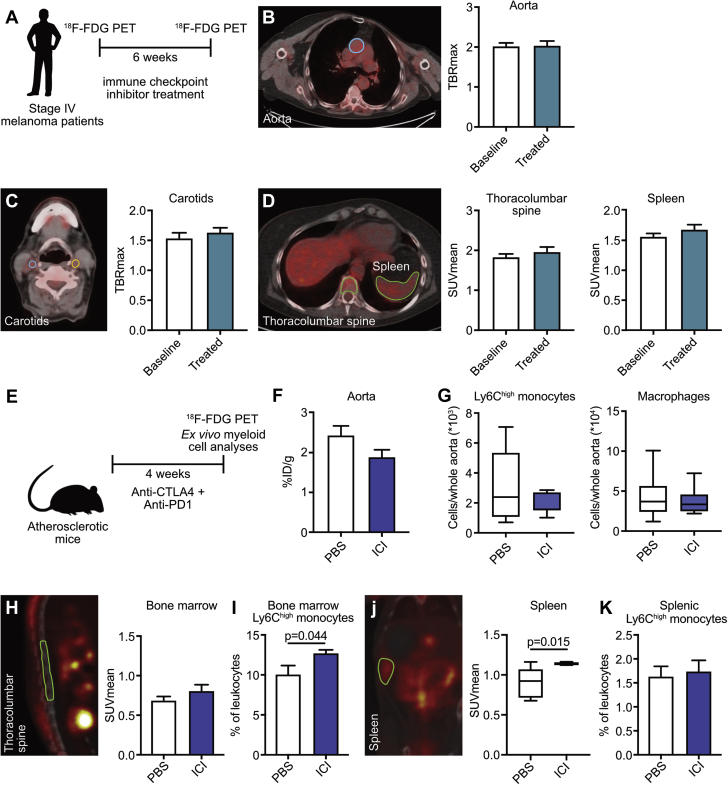
Figure 1.
18F-FDG PET–Based Analysis of ICI Therapy in Humans and Apoe–/– Mice
(A to D) Ten stage IV melanoma patients underwent 2-deoxy-2-[fluorine-18]fluoro-D-glucose (18F-FDG) positron emission tomography–computed tomography (PET/CT) imaging at baseline and 6 weeks after the start of immune checkpoint inhibitor (ICI) therapy. (A) Schematic overview of experimental design. (B) Representative PET/CT image and maximum target-to-background ratio (TBRmax) of the thoracic aorta before (baseline) and after ICI therapy (treated). (C) Representative PET/CT image and TBRmax of common carotid arteries before and after ICI therapy. (D) Representative PET/CT image and mean standardized uptake value (SUVmean) of the thoracolumbar spine and the spleen, before and after ICI therapy. (E) Schematic overview of experimental design. Apoe–/– mice (n = 8) were treated with anti-CTLA-4 (anti-cytotoxic T lymphocyte-associated antigen-4)/anti-PD-1 (anti-programmed cell death protein-1) antibodies or phosphate-buffered saline (PBS) for 4 weeks and subjected to 18F-FDG PET/CT imaging. (F) Ex vivo quantification of 18F-FDG accumulation in the aorta. (G) Flow cytometry analysis of Ly6Chight monocytes and macrophages in the aorta. (H) Representative PET/CT image and SUVmean of the thoracolumbar spine. (I) Flow cytometry analysis of Ly6Chigh monocytes in the bone marrow. (J) Representative PET/CT image and SUVmean of the spleen. (K) Flow cytometry analysis of Ly6Chigh monocytes in the spleen. For all graphs, bars represent mean ± SEM.