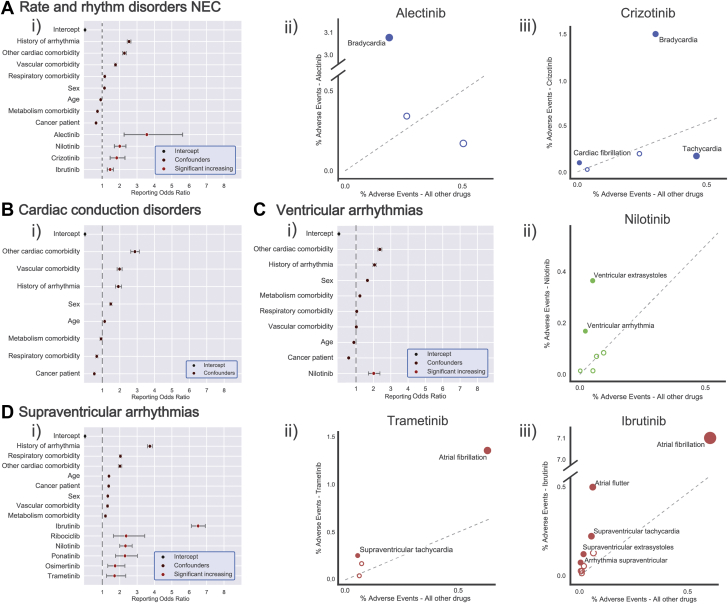
Figure 3.
Odds Ratios for Protein Kinase Inhibitors With Regards to Related Arrhythmias
All protein kinase inhibitors were assessed for elevated reporting of arrhythmias grouped under Medical Dictionary for Regulatory Activities (MedDRA) high level terms (HLTs): “rate and rhythm disorders” (A), “cardiac conduction disorders” (B), “ventricular arrhythmias” (C), and “supraventricular arrhythmias” (D). For each analysis, compounds with significantly elevated ROR after multiple-testing correction are presented along with confounder effects. Confounder effects with ROR < 1 does not imply a protective effect. For compounds with significantly elevated reporting, a post hoc analysis assessed for enrichment of underlying PTs to identify recurrent etiological themes. Significantly enriched or under-represented terms are labelled and shown with filled-in markers. The size of each marker is proportional to total number of reports for this form of arrhythmia for this drug. (Ai) Alectinib, nilotinib, crizotinib, and ibrutinib were associated with elevated rate and rhythm disorder reporting. The alectinib (Aii) and crizotinib (Aiii) signals were primarily driven by reporting of Bradycardia. (Bi) No protein kinase inhibitors were associated with elevated cardiac conduction disorder reporting. (Ci) Nilotinib was associated with elevated ventricular arrhythmia reporting. (Cii) This signal was primarily driven by reporting of ventricular extrasystoles and nonspecific ventricular arrhythmias. (D) With the exception of idelalisib, all other significant protein kinase inhibitors detected in the AF analysis were associated with elevated supraventricular arrhythmia reporting. Trametinib (Dii) and ibrutinib (Diii) signals were primarily driven by reporting of AF, and to a lesser extent by closely related terms (e.g., supraventricular tachycardia and atrial flutter). Abbreviations as in Figure 1.