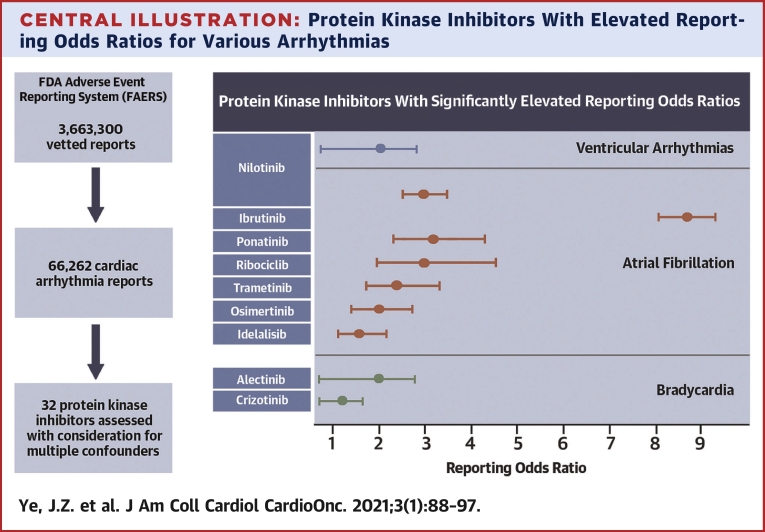
Central Illustration.
Protein Kinase Inhibitors With Elevated Reporting Odds Ratios for Various Arrhythmias
Logistic regression analysis of 3,663,300 U.S. Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) reports (66,262 cardiac arrhythmia reports) that assessed the likelihood of developing arrhythmia. After controlling for confounding variables, 9 protein kinase inhibitors have significantly increased likelihood of being reported for arrhythmia, including ibrutinib, ponatinib, nilotinib, ribociclib, trametinib, osimertinib, and idelalisib for atrial fibrillation specifically. The 95% confidence intervals are skewed due to being in exponential space, and are not corrected for multiple hypothesis testing.