Erdheim-Chester disease (ECD) is classified as an inflammatory myeloid neoplasia that has an unknown origin and low incidence (∼600 cases described in the literature) (1,2). ECD is multisystemic, affecting the bones, skin, ocular tissue, lungs, brain, pituitary gland, and other tissues and organs. The most frequent symptom is bone pain (2). Cardiovascular involvement is common, present in more than one-half of patients, and frequently asymptomatic; however, it is associated with a worse prognosis (1,2). Cardiac tamponade is not commonly reported in the literature (3, 4, 5). We present a rare case of symptomatic pericardial disease as the first clinical presentation of ECD.
Case Report
A 55-year-old woman with no prior comorbidities presented to the emergency department with progressive dyspnea with minimal exertion, orthopnea, and lower extremity edema over the last 3 months. These symptoms were associated with atypical chest pain, which had worsened 1 day earlier. She also reported recent episodes of syncope. Her initial blood pressure was 80/60 mm Hg, and electrocardiography showed sinus tachycardia with low QRS voltage. Although the patient was afebrile and her white blood cell count was 13,260/mm3 (neutrophils 66.5%, lymphocytes 24.5%, and monocytes 9.1%), her C-reactive protein was moderately elevated (17 mg/dl). Transthoracic echocardiography revealed a large pericardial effusion and echocardiographic signs of cardiac tamponade.
Pericardiocentesis was performed, and 600 ml of serous fluid was drained. The pericardial fluid analysis was compatible with an inflammatory transudate, and no malignant cells were found. Results of the bacterial cultures, fungal cultures, and acid-fast bacilli tests were negative. A pericardial biopsy sample showed a slight inflammatory pericarditis.
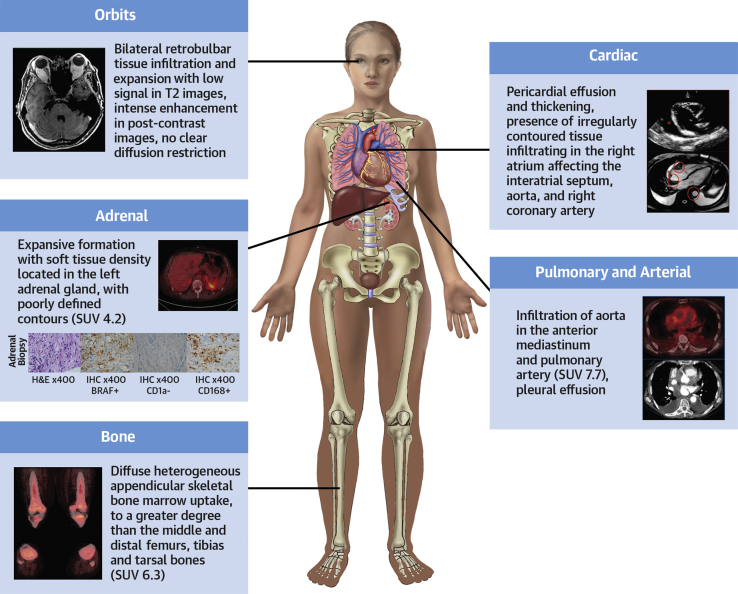
An etiological investigation was therefore conducted. Cardiac magnetic resonance (CMR) imaging showed right atrial (RA) enlargement and irregularly contoured tissue that had infiltrated the RA wall, affecting the interatrial septum, aorta, right coronary artery, and vena cava (Figure 1). A slight reduction in myocardial perfusion and heterogeneous late gadolinium enhancement in the RA wall were observed. These findings overall suggested an infiltrative disorder; the differential diagnosis included non-Langerhans cell histiocytosis (LCH) and cardiac lymphoma.
Figure 1.
Multimodality Imaging Approach Showing the Systemic Involvement of ECD
The graphic summarizes the findings in the reported case, highlighting the main organs and systems affected by Erdheim-Chester disease (ECD) (involvement of the heart, pericardium, pleura, aorta, pulmonary artery, adrenal, orbits, and bones). – = negative; + = positive; IHC = immunohistochemistry; H&E = hematoxylin and eosin; SUV = standardized uptake value.
As confirmation of a diagnosis of non-LCH, an adrenal biopsy sample revealed histiocytic proliferation. Immunohistochemical analysis showed positivity for CD68, CD163, S-100 protein (focal), and BRAF, and negativity for CD1A. This result was compatible with ECD. 18F-Fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) showed bone and thoracic involvements characterized by high uptake of 18F-fluorodeoxyglucose (standardized maximum uptake value 6.3 on the femur) in the mediastinal region and bones. The patient was referred to the hematology service for evaluation and discussion of therapy. Treatment with interferon and zoledronic acid was started.
Discussion
This is a rare case of cardiac tamponade as the initial manifestation of ECD. ECD, a member of the L group of histiocytic disorders, is a systemic disease that is largely driven by mutations of the mitogen-activated protein kinase/extracellular signal–regulated kinase pathway, with a high frequency of BRAFV600E mutations. ECD is a non-LCH disease characterized by abnormal production and accumulation of histiocytes in organs and tissues (6,7). Cardiac involvement, which is common in ECD, results in a worse prognosis and is an important cause of mortality among these patients; thus, prompt recognition and treatment are critical (3,4,7).
The diagnosis of ECD is made via distinctive histopathological findings in the appropriate clinical and radiological context, which requires a multimodality diagnostic approach. Laboratory examinations should include evaluation of blood count, renal and liver function, dosage of lactate dehydrogenase, C-reactive protein level, and endocrine analysis.
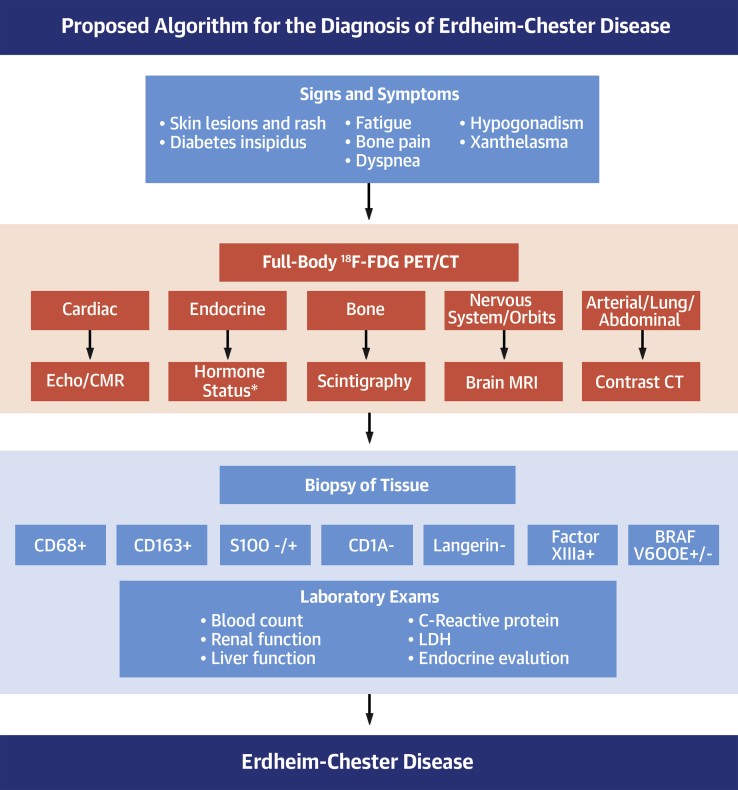
To obtain a correct diagnosis, brain magnetic resonance imaging, 18F-FDG PET/CT scans, CMR imaging, and, in some cases, contrast tomography is performed (1,2,6). In this complex disease, with broad systemic involvement, it is essential that the clinicians know the main features so that they can perform an early and a prompt diagnostic investigation. In Figure 2, we propose a diagnostic algorithm for ECD.
Figure 2.
Proposed Algorithm for the Diagnosis of Erdheim-Chester Disease
The first step for the correct diagnosis is the recognition of the signs and symptoms. According to the system involved, there is a need for complementary examinations. The diagnosis is confirmed with results of a biopsy. ∗Morning urine and serum osmolality, follicle-stimulating hormone, and luteinizing hormone with testosterone (male subjects) and estradiol (female subjects), corticotropin with morning cortisol, thyrotropin and free T4, prolactin, and insulin-like growth factor-I. 18F-FDG PET/CT = 18F-fluorodeoxyglucose positron emission tomography/computed tomography; – = negative; + = positive; CMR = cardiac magnetic resonance; CT = computed tomography; Echo = echocardiography; LDH = lactate dehydrogenase; MRI = magnetic resonance imaging.
The mean age at diagnosis of ECD is between 45 and 60 years, and there is a slight male predominance (3,6,8). The cardiovascular manifestations of the disease are multiple and may involve the myocardium, pericardium, cardiac valves, aorta, coronary arteries, and conduction system (3). Circumferential soft-tissue sheathing of the thoracic and abdominal aorta is present in ∼56% of patients, and mural pseudotumoral infiltration is present in up to 40% to 70% of patients, visualized clearly on CMR imaging (1,2,6). Renovascular hypertension may develop when renal arteries are involved and could require treatment with percutaneous intervention. Coronary disease can result in myocardial infarction (7). Diffuse infiltration of the myocardium or interatrial septum has been described, occasionally leading to heart failure (2,4).
The prevalence of pericardial disease in these patients varies, ranging from 20% to 44% (3,4,9). In a cohort of 24 patients, Ghotra et al. (3) observed that 13% presented with diffuse pericardial infiltration, and 4% had pericardial thickening/calcification and no pericardial effusion. Berti et al. (9), however, reported pericardial effusion in 24% of patients. Symptomatic pericardial disease with hemodynamic impairment is rarely reported (3, 4, 5).
Recognition of the clinical features of ECD is useful in differentiating it from other histiocytic neoplasms such as LCH and Rosai-Dorfman disease (RDD). The cardiac involvement characterized by the infiltration of peri-aortic and infiltration of the right atrium, interatrial septum, and left ventricle is described in RDD in <5% of cases (6). Cardiac involvement is also rarely reported in LCH. In most cases, histopathological characteristics help to confirm the diagnosis. Specific positive immunohistochemical markers are usually detected in the lesional histiocytes of ECD, RDD, and LCH that are not otherwise present in the background reactive infiltrate (6).
Treatment of ECD is not fully established and is mainly based on the use of interferon-α, corticosteroids, cytotoxic chemotherapies, the kinase inhibitors vemurafenib or imatinib, radiotherapy, and surgery, based on the systems involved (2). Interferon is the most prescribed treatment and is the main therapy for BRAF-negative patients. Vemurafenib is an inhibitor of mutated BRAF that is increasingly being used and growing as a promising therapy for ECD (10). In a retrospective study of 24 patients, mortality was 17% at a median follow-up of 5.5 years (3). However, other studies have reported a 5-year survival rate of ∼60% with a poor prognosis with heart involvement (1,4,7,10).
Conclusions
The case reported here is a rare presentation of ECD, a non-LCH, complicated by cardiac tamponade. In cases with early involvement of the heart, prognosis is poor. Cardiologists’ knowledge about ECD is essential to allow for prompt diagnosis and management.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: CardioOncologyauthor instructions page.
References
- 1.Costa I., Abdo A.N.R., Bittar C.S. Cardiovascular manifestations of Erdheim-Chester's disease: a case series. Arq Bras Cardiol. 2018;111:852–855. doi: 10.5935/abc.20180218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diamond E.L., Dagna L., Hyman D.M. Consensus guidelines for the diagnosis and clinical management of Erdheim-Chester disease. Blood. 2014;124:483–492. doi: 10.1182/blood-2014-03-561381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghotra A.S., Thompson K., Lopez-Mattei J. Cardiovascular manifestations of Erdheim-Chester disease. Echocardiography. 2019;36:229–236. doi: 10.1111/echo.14231. [DOI] [PubMed] [Google Scholar]
- 4.Haroche J., Amoura Z., Dion E. Cardiovascular involvement, an overlooked feature of Erdheim-Chester disease: report of 6 new cases and a literature review. Medicine (Baltimore) 2004;83:371–392. doi: 10.1097/01.md.0000145368.17934.91. [DOI] [PubMed] [Google Scholar]
- 5.Buono A., Bassi I., Santolamazza C. Getting to the heart of the matter in a multisystem disorder: Erdheim-Chester disease. Lancet. 2019;394:e19. doi: 10.1016/S0140-6736(19)31787-8. [DOI] [PubMed] [Google Scholar]
- 6.Goyal G., Young J.R., Koster M.J. The Mayo Clinic Histiocytosis Working Group Consensus Statement for the Diagnosis and Evaluation of Adult Patients With Histiocytic Neoplasms: Erdheim-Chester disease, Langerhans cell histiocytosis, and Rosai-Dorfman disease. Mayo Clin Proc. 2019;94:2054–2071. doi: 10.1016/j.mayocp.2019.02.023. [DOI] [PubMed] [Google Scholar]
- 7.Mazor R.D., Manevich-Mazor M., Shoenfeld Y. Erdheim-Chester disease: a comprehensive review of the literature. Orphanet J Rare Dis. 2013;8:137. doi: 10.1186/1750-1172-8-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavalli G., Guglielmi B., Berti A., Campochiaro C., Sabbadini M.G., Dagna L. The multifaceted clinical presentations and manifestations of Erdheim-Chester disease: comprehensive review of the literature and of 10 new cases. Ann Rheum Dis. 2013;72:1691–1695. doi: 10.1136/annrheumdis-2012-202542. [DOI] [PubMed] [Google Scholar]
- 9.Berti A., Ferrarini M., Ferrero E., Dagna L. Cardiovascular manifestations of Erdheim-Chester disease. Clin Exp Rheumatol. 2015;33:S155–S163. [PubMed] [Google Scholar]
- 10.Estrada-Veras J.I., O'Brien K.J., Boyd L.C. The clinical spectrum of Erdheim-Chester disease: an observational cohort study. Blood Adv. 2017;1:357–366. doi: 10.1182/bloodadvances.2016001784. [DOI] [PMC free article] [PubMed] [Google Scholar]