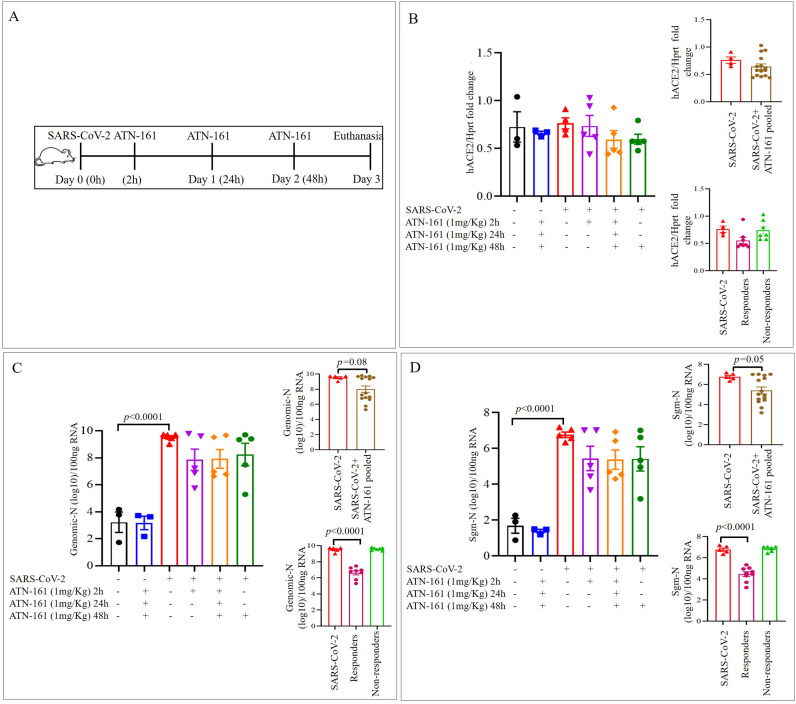
Fig. 1.
ATN-161 reduces the Genomic-N and Sub genomic N (Sgm—N) viral load in SARS-CoV-2 infected K18-hACE2 mice lung tissue
Schematic overview of experimental timeline for K18-hACE2 mice (A) 10-week old male K18-hACE2 transgenic mice administered saline (black bar) or ATN-161 (1 mg/kg, blue bar) intravenously (via retro-orbital). Mice were inoculated via the intranasal route with Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (2 × 105 TCID50) + Saline (i.v. by retroorbital rout) (Red bar). ATN-161 (1 mg/Kg) treatment was administered at 3 different time periods, SARS-CoV-2 + ATN-161-2 h post infection (purple bar), SARS-CoV-2 + ATN-161-daily (2 h, 24 h and 48 h) administration (orange bar), and SARS-CoV-2 + ATN-161-48 h administration (green bar) post SARS-CoV-2 intranasal inoculation. Panels B, C, and D top inserts with brown bar represents SARS-CoV-2 + ATN-161 all treatment groups pooled data; Panels B, C, and D bottom insert with pink bar represents responding mice and light green bar represents nonresponding with SARS-CoV-2 + ATN-161 (either 2, daily or 48 h) treatment. 3 days post infection (3 dpi) the mice were euthanized, and RNA isolated from the left lungs by Trizol method for qRT-PCR (B) hACE2 expression in lungs of K18-hACE2 mice (C) viral genomic-N (Total-N) and (D) sub genomic-N mRNA (sgm-—N). Experimental groups are divided into 6 groups. Saline n = 3; ATN-161 n = 3; SARS-CoV-2 (5 mice for vehicle, 5 mice for each 3 ATN-161 groups). Data are presented as mean ± SEM. P values represent saline vs SARS-CoV-2 + vehicle and SARS-CoV-2 + vehicle vs SARS-CoV-2 + ATN-161 treatment groups. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)