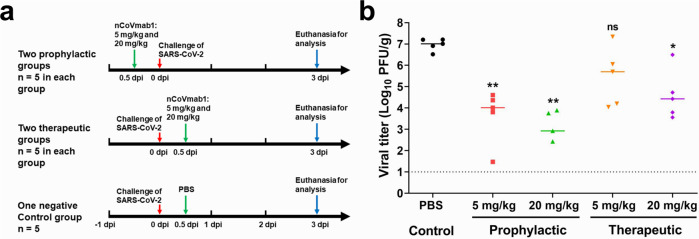
Fig. 3. Protective efficacy of nCoVmab1 in vivo.
a Experimental route of prophylactic and therapeutic tests of nCoVmab1 in hACE2 transgenic mice. hACE2 transgenic mice were intraperitoneally injected with low (5 mg/kg, n = 5 mice/group) and high (20 mg/kg, n = 5 mice/group) doses of nCoVmab1 12 h before or 12 h after SARS-CoV-2 infection. PBS was used as a negative control (n = 5 mice/group). b The viral titers in the lungs from different groups were determined at 3 dpi by plaque assay (dashed line represents the detection limit). The median values were presented. In the high-dose prophylactic group, one mouse had a viral titer below the detection limit. Statistical significance was measured by using one-way ANOVA with Dunnett’s multiple comparisons, not significant [ns]: p > 0.05, *p < 0.05, **p < 0.01. For the prophylactic experiment in b, p value between the PBS group and the low dose group is 0.0096; p value between the PBS group and the high dose group is 0.0095. For the therapeutic experiment in b, p value between the PBS group and the low dose group is 0.2007; p value between the PBS group and the high dose group is 0.0150.