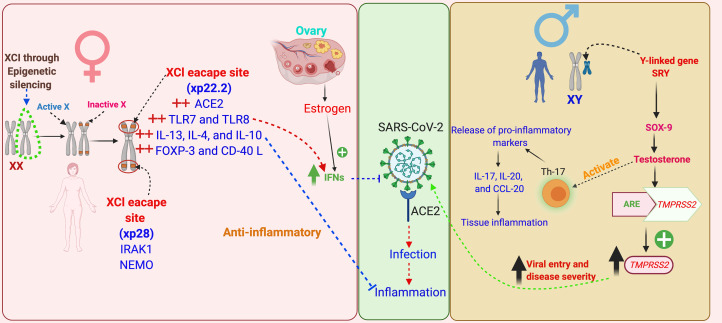
Fig. 5.
Schematic representation of the role of evolution, genetics, epigenetic mechanisms and sex hormones in sex-based differences in COVID-19 outcomes. Females bear two X chromosomes, of which one gets inactivated during oogenesis through epigenetic silencing of the genes – this phenomenon is known as ‘X-chromosomal inactivation’ (XCI). Some of the X-linked genes escape XCI, among these are the key immune functions genes, such as ACE2, TLRs-7, -8, ILs-4, -10 and -13, FoxP3, CD40L, IRAK1 and NEMO, thus these genes express in females in double dosage in comparison with males. These immune genes not only prime the females for a stronger immune response against infectious agents, but also prevent against hyper-inflammatory responses, such as CS. Apart from this, certain immune genes have sex hormone-specific regulatory elements in their promoter region modulating their expression, such as oestrogen has for IFN, and TMPRSS2 has for TMPRSS2. A marked influence of sex hormones on the formation of cytokines has also been noted, such as testosterone induces greater syntheses of Th-17 cells and in turn release of pro-inflammatory markers in males, such as IL-17, IL-20 and CCL-20 – thus favour hyper-inflammatory responses and in turn poor clinical outcomes. XCI, X chromosome inactivation; ACE2, angiotensin-converting enzyme 2; TMPRSS2, transmembrane protease, serine 2; ARE, androgen receptor element; SRY, sex-determining region Y; SOX-9, SRY-box transcription factor-9; TLRs, Toll-like receptors; IFN, interferon; ILs, interleukins; FoxP3, forkhead box P3; CD40L, cluster of differentiation 40L; CCL-20, chemokine (C–C motif) ligand-20; IRAK1, interleukin-1 receptor-associated kinase 1; NEMO, NF-κB essential modulator.