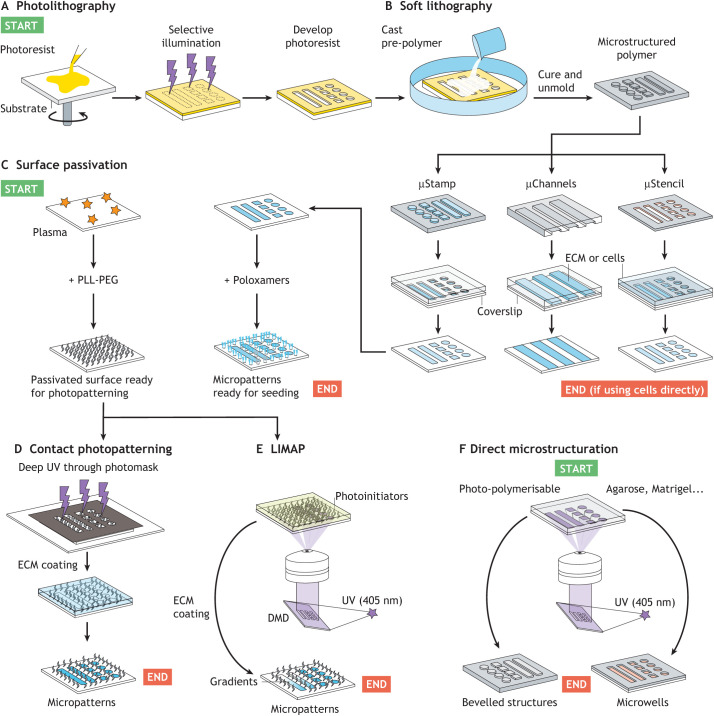
Fig. 2.
Micropattern production methods. (A-F) There exists a broad range of strategies for making micropatterns. Most require a combination of generic procedures indicated in the following panels. The start and end point of different workflows is shown in green and red, respectively. In photolithography (A) the coating of the photoresist is generally performed via spin-coating. Selective illumination can be achieved using a photomask, lasers or a digital micromirror device (DMD). The microstructured photoresist can then be used as a mould for soft lithography (B). PDMS is often used in soft lithography as this polymer self-seals reversibly when placed in contact with another smooth substrate, permitting watertight stencilling and channels to direct the spatial deposition of extracellular matrix (ECM) molecules or cells. If ECM molecules are deposited, surface passivation is needed before cell seeding and can be done using poloxamers (a polymer that adsorbs preferentially on hydrophobic surfaces to form a monolayer of cell repellent molecules) (C, right). Another popular passivation method includes plasma treatment (to activate the surface by ripping-off electrons from the material) followed by adsorption of PLL-PEG (C, left). Selective degradation is then performed with either deep UV through a photomask (D) or using light-induced molecular adsorption patterning (LIMAP) (E), in which ECM density scales linearly with the dose of light allowing for gradient micropatterns. Finally, selective photopolymerisation or photoscission can be performed to create 3D microstructured hydrogels (F). Microstructuration and patterning can be combined to create complex cell environments or microdevices to probe cellular properties (Box 2).