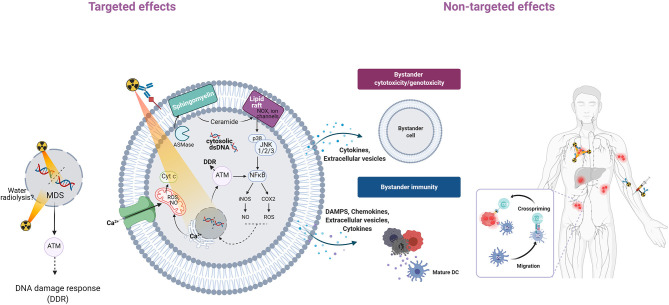
Figure 1.
Overview of targeted and non-targeted effects of radiation. Alpha-particle irradiation of a cell population can induce targeted effects (in cells hit directly by particles) and non-targeted effects (in non-irradiated cells). Non-targeted effects can be detected at short distance (bystander effects) or at long distance (systemic effects). Genomic instability, another class of non-targeted effects, is not described here. In irradiated cells (targeted effects), alpha particles induce DSBs and non-DSB clustered DNA lesions (MDS) that are detected by ATM and activate the DNA damage response (11, 12). Alpha-particle irradiation of the cell membrane generates lipid peroxidation products (4-HNE, 4-hydroxy-2-nonenal; MDA, malondialdehyde) from polyunsaturated fatty acids (PUFA) (43). Alpha-particle irradiation can also activate acid sphingomyelinase (ASMase) and this leads to the rapid formation of ceramide through hydrolysis of sphingomyelin, a cell membrane phospholipid (38, 42). Ceramide-enriched large domains (lipid rafts) are formed by aggregation of ceramide, leading to activation of the mitogen-activated protein kinase (MAPK) pathway and its downstream effector, nuclear factor kappa B (NF-κB) (41). NF-κB induces the transcription of target genes, such as those encoding cytokines, COX-2, and inducible nitric oxide synthase (iNOS), followed by production of ROS and nitric oxide (NO) that contribute to oxidative stress (44). Irradiation can also increase the intracellular Ca2+ level (45) through release from the endoplasmic reticulum via calcium release mechanisms (46). Ca2+ can in turn activate protein kinase C, the MAPK pathway and transcription factors (NF-κB, AP1) that promote various downstream pathways (iNOS, COX-2). Mitochondria also are affected by alpha-particle irradiation (47–52). Ca2+ can be taken up by mitochondria, leading to ROS and RNS increase, mitochondrial DNA damage, altered ATP synthesis, mitochondrial depolarization, and release of cytochrome C and caspase 3. Mitochondrial fission also has been observed. Targeted cells can communicate with bystander cells through gap junctions or through the release of soluble factors. Extracellular vesicles, including exosomes, containing nucleic acids, lipids and proteins, might be released, and contribute to bystander immunity. Systemic effects may involve the immune system through the release of DAMPs that are recognized by antigen-presenting cells (e.g., dendritic cells, DC, that present antigenic peptides to CD4+ and CD8+ T lymphocytes for immune response activation). Altogether these effects contribute to tumor cell death.