Figure 4.
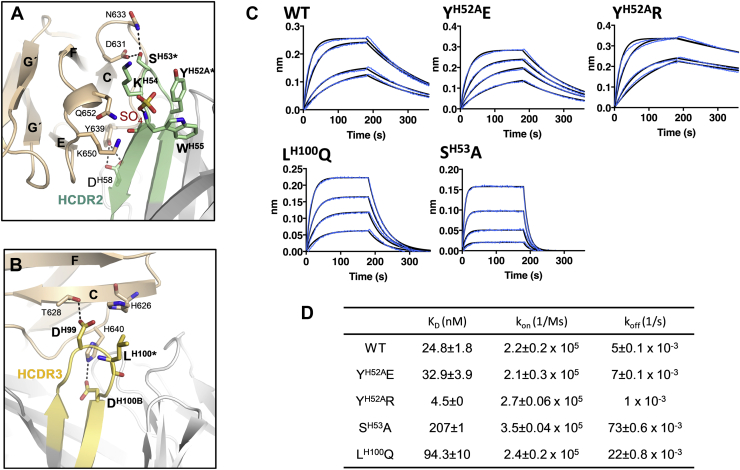
Mutations in the m971 paratope impact binding affinity to CD22.A, interactions of the m971 HCDR2 with the CD22 d7 domain (wheat). Sulfate ion site III (red and yellow sticks) forms an H-bond with the amide backbone of WH55. B, interactions of the m971 HCDR3 with the CD22 d7 domain (wheat). Dashed black lines in panels A and B represent polar contacts. Mutated residues in HCDR2 and HCDR3 are highlighted with an asterisk. C, biolayer interferometry (BLI) data showing binding of WT and mutant m971 Fabs (200, 100, 50, and 25 nM concentrations) to CD22d1–d7. Raw experimental data are in blue, and fitted curves for binding kinetics determination are in black. D, KD, kon, and koff values describing the binding of WT and mutant m971 Fabs to CD22d1–d7. KD, kon, and koff mean values are presented with their SEM derived from two independent BLI measurements. Fab, fragment antigen-binding; HCDR, heavy-chain complementarity-determining region.