Figure 1. Immobilization induces a rapid and progressive decrease in the relative rate of protein synthesis.
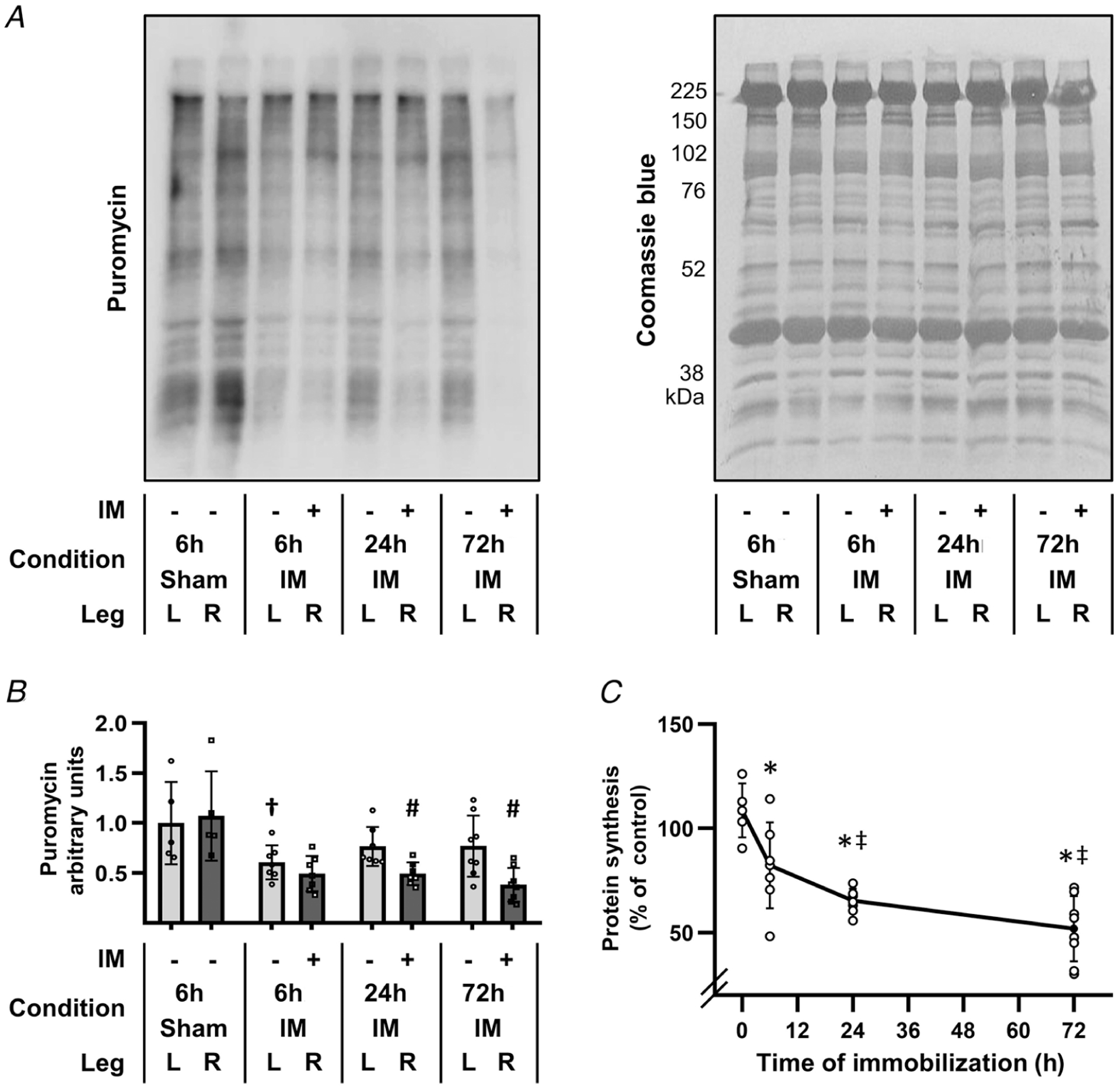
The right hindlimbs of mice were subjected to immobilization (IM) for 6, 24 or 72 h, or subjected to a 6 h sham control condition. All mice were injected with puromycin at 30 min prior to muscle collection for the measurement of protein synthesis. The plantar flexor muscles from both the left (L) and the right (R) hindlimbs were collected and subjected to western blot analysis for puromycin-labelled peptides (i.e. the relative rate of protein synthesis). A, representative western blot of puromycin-labelled peptides, and the subsequent Coomassie blue stain of total protein. B, graph showing the relative amount of puromycin-labelled peptides that were detected in the various conditions. C, graph illustrating the effect of immobilization on protein synthesis, which was determined by calculating the ratio between the amount of puromycin-labelled peptides in the R (immobilized or sham) muscle by the amount in the contralateral L (control) muscle. All R/L ratios were expressed as a percentage of the mean R/L ratio obtained in the 6 h sham control group (i.e. 0 h immobilization). Values in B and C are presented as the mean ± SD from n = 5–8 per group. Significantly different from, †time- and limb-matched sham control, #time-matched contralateral control, *0 h immobilization, and ‡6 h immobilization. Significance was determined by (B) repeated measures two-way ANOVA followed by planned comparison (interaction P < 0.001), or (C) one-way ANOVA followed by Student–Newman–Keuls post hoc analysis, P ≤ 0.05.
