Figure 3. Experimental workflow for mapping the proteomic and phosphoproteomic alterations that occur in response to immobilization.
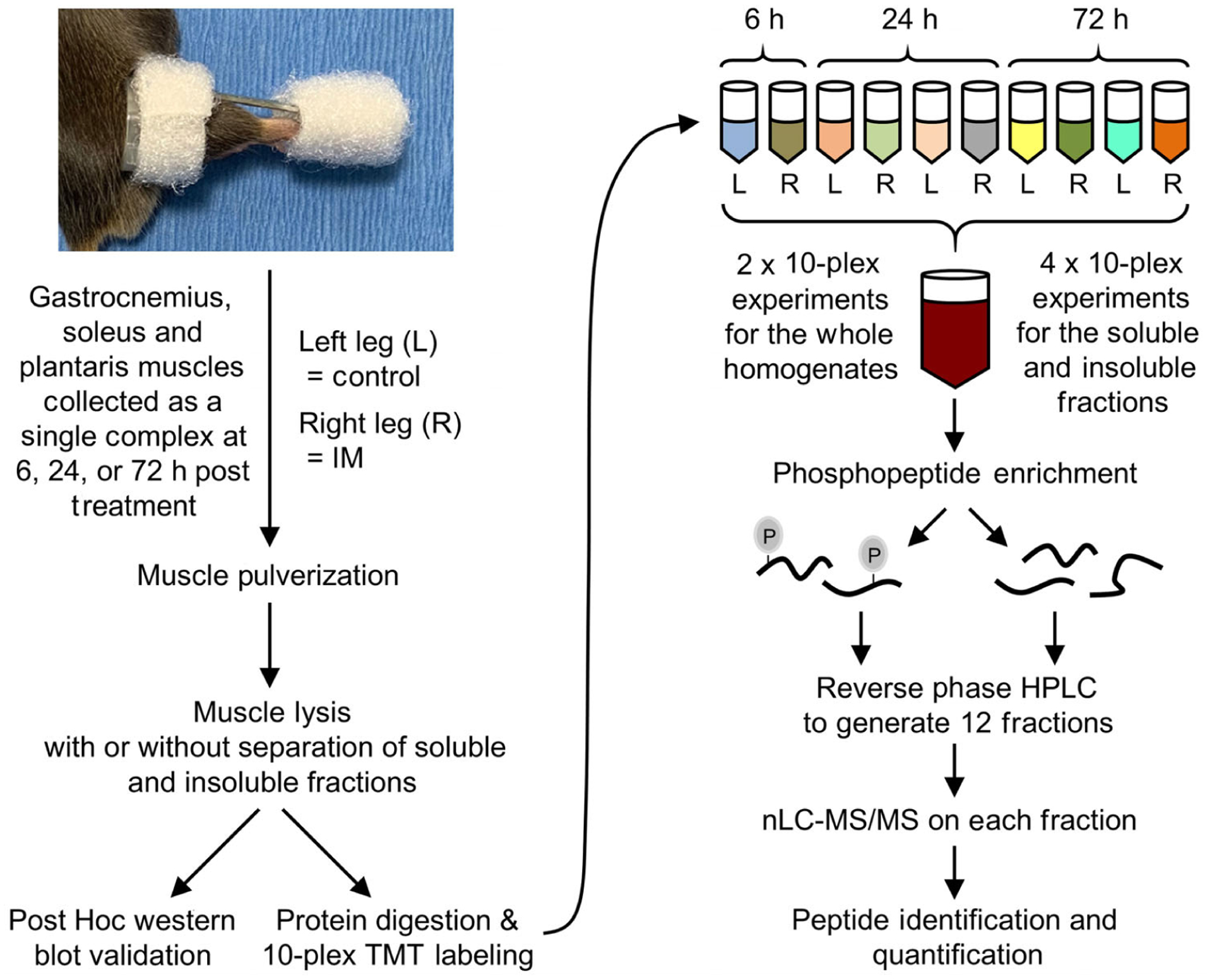
The right hindlimbs of mice were subjected to immobilization (IM) for 6, 24 or 72 h. The plantar flexor muscles (i.e. the gastrocnemius, soleus and plantaris) from both the left control (L) and right (R) hindlimbs were collected as a single complex and subjected to mass spectrometry (MS). Specifically, the muscles were pulverized and lysed, and then proteins from the whole homogenate, or soluble and insoluble fractions, were tryptically digested (see Methods for details). The resulting peptides from each sample were labelled with different tandem mass tags (TMT) and mixed to yield 10-plex pooled samples. Immobilized metal affinity chromatography was then used to enrich phosphopeptides and a total of 12 fractions for phosphopeptides and 12 fractions for the unbound (non-phospho) peptides was generated by reversed-phase HPLC. All fractions were analysed by nano-liquid chromatography-tandem MS spectrometry (nLC-MS/MS) and the relative quantity of the phosphopeptides and non-phosphorylated peptides in each sample was determined by the TMT reporter ions in the MS spectra.
