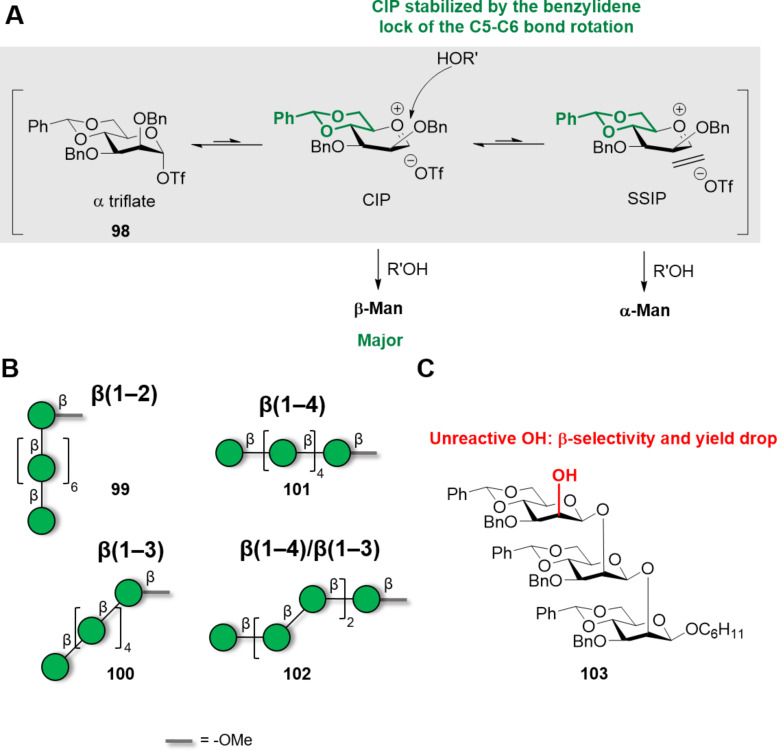
Figure 7.
PG stereocontrol strategy to obtain β-mannosides. A) The mechanism of the β-mannosylation reaction is thought to proceed through a contact ion pair (CIP) in which the triflate anion shields the α-face favoring the nucleophile attack from the β-face. The solvent separated ion pair (SSIP) scenario is disfavored due to the benzylidene group locking the C5–C6 bond in the tg conformation which destabilizes the oxocarbenium ion. For a thorough explanation of the mechanism we refer to the original publication [289]. B) Examples of β-mannosides synthesized using the 4,6-O-benzylidene directed β-mannosylation strategy. C) Chain elongation beyond the trisaccharide level for β(1–2)-analogues presented a significant drop in yield and β-stereoselectivity due to the conformation of acceptor 103.