Fig. 7. Proposed Model of IKK:NF-κB:Notch Signaling Control by SLX4IP and RAP1.
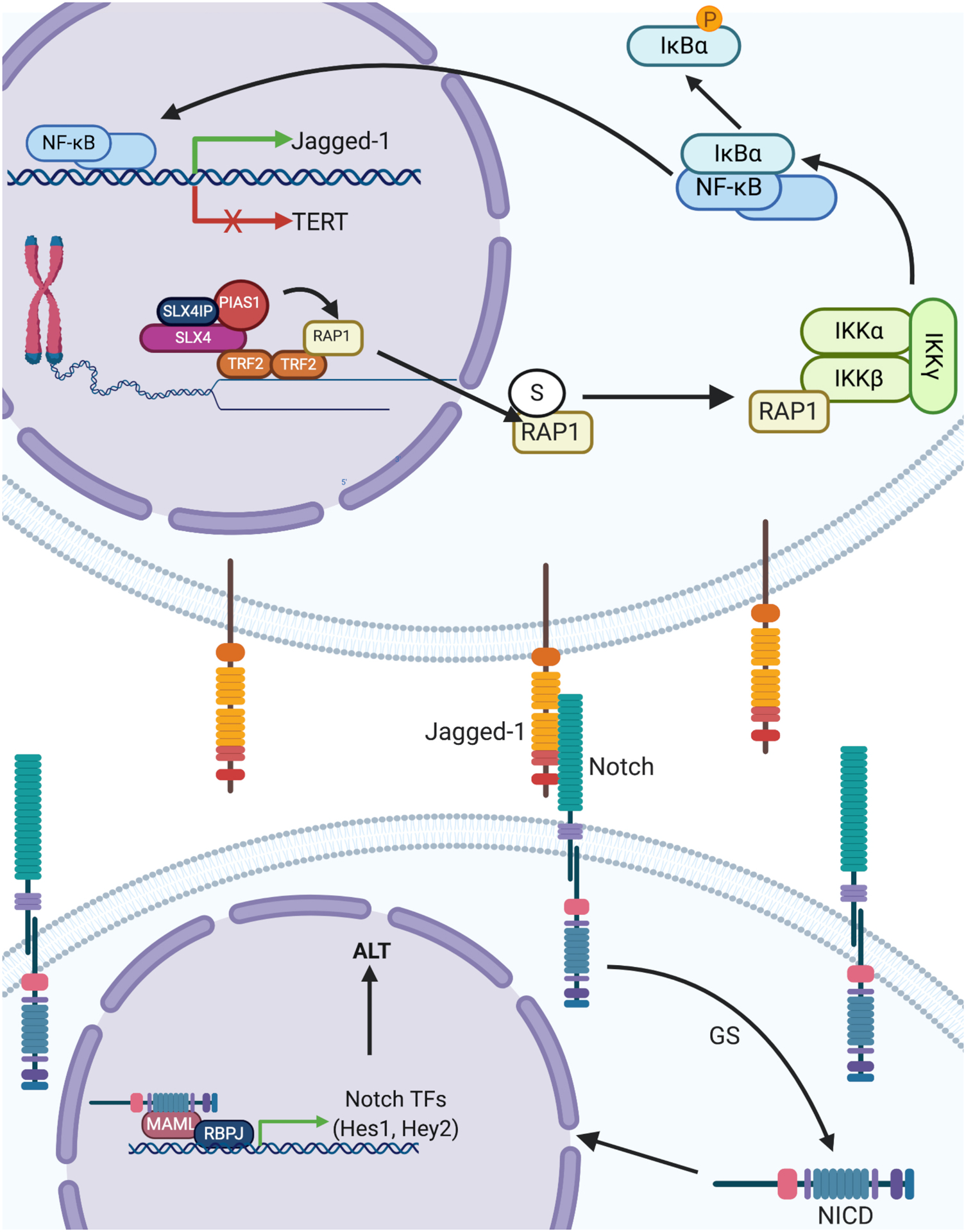
SLX4IP facilitates recruitment of PIAS1 to the SLX4 scaffold and promotes SUMOylation (S) of RAP1. SUMOylated RAP1 is exported from the nucleus to the cytoplasm, where it interacts with IKKβ (as part of the heterotrimeric IKK complex), stimulating NF-κB nuclear translocation via IκBα phosphorylation and degradation. Once in the nucleus, NF-κB increases Jagged-1 transcription while simultaneously suppressing TERT. Jagged-1 binds to Notch receptors on neighboring cells, which liberates the Notch intracellular domain (NICD) through activation of the γ-secretase (GS) pathway. NICD is a transcription factor that recognizes its targets by binding with additional transcriptional co-activators (RBPJ and MAML). These targets comprise a host of transcription factors (for example, Hes1 and Hey2) that directly coordinate the cellular Notch response. This response includes the modulation of ALT-associated gene expression as well as other features of ALT, such as APB formation. In the absence of SLX4IP, RAP1 fails to be SUMOylated and bind to IKKβ, which diminishes both NF-κB and Notch signaling, thereby removing repression of TERT and dampening Notch-driven activation of ALT. In this way, SLX4IP promotes ALT, and we hypothesize that it controls telomere plasticity through its role in directing PIAS1-mediated SUMOylation of RAP1.
