FIGURE 4.
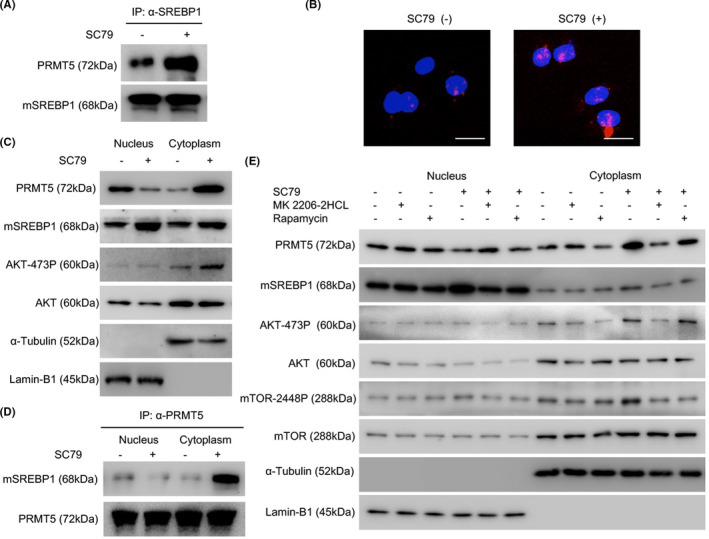
Activation of protein kinase B (AKT) promotes the interaction between endogenous protein arginine methyltransferase 5 (PRMT5) and mature sterol regulatory element‐binding protein 1 (mSREBP1) by changing PRMT5 subcellular localization. A, After culture of NCI‐H1299 cells in medium without FBS for 24 h, SC79 (4 µg/mL) was added (DMSO as control), and the cells were harvested at 24 h. Cell lysates were immunoprecipitated with anti‐SREBP1 Ab, and the mSREBP1 expression level was then normalized. An anti‐PRMT5 Ab was used to analyze the interaction between mSREBP1 and PRMT5. B, After the culture of NCI‐H1299 cells in medium without FBS for 24 h, SC79 (4 µg/mL) was added (DMSO as control), and a proximity ligation assay was carried out at 24 h. Scale bar = 25 µm. C, After culture of NCI‐H1299 cells in medium without FBS for 24 h, SC79 (4 µg/mL) was added (DMSO as control) for 24 h. The cytoplasmic and nuclear fractions were then separated and immunoblotted with Abs against SREBP1, PRMT5, Ser473‐phosphorylated AKT (AKT‐473P), or AKT. α‐Tubulin was used as a cytoplasmic fraction loading control while Lamin‐B1 served as the nuclear fraction loading control. D, After culture of NCI‐H1299 cells in medium without FBS for 24 h, SC79 (4 µg/mL) was added (DMSO as control) for 24 h. The cytoplasmic and nuclear fractions were then separated and immunoprecipitated with anti‐PRMT5 Ab, and the PRMT5 expression levels were then normalized. The anti‐SREBP1 Ab was used to analyze the mSREBP1 and PRMT5 interaction in the cytoplasm and nucleus. E, After culture of NCI‐H1299 cells in medium without FBS for 24 h, SC79 (4 µg/mL) was added (DMSO as control) for 12 h, MK2206‐2HCL (100 nmol/L; Selleck) or rapamycin (1 µmol/L; Selleck) was then added (DMSO as control) for 24 h. The cytoplasmic and nuclear fractions were then separated and immunoblotted with Abs against SREBP1, PRMT5, mTOR, mTOR‐2448P, AKT‐473P, or AKT. α‐Tubulin was used as a cytoplasmic fraction loading control. Lamin‐B1 served as the nuclear fraction loading control
