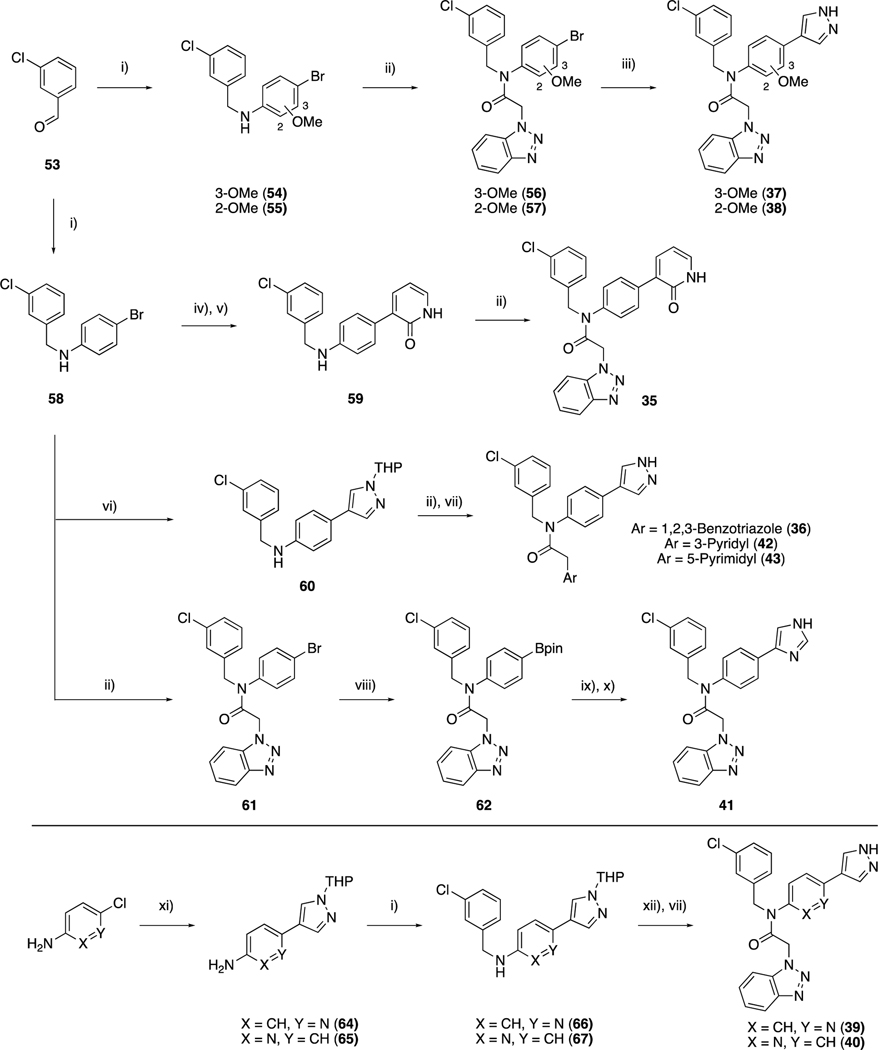
Scheme 3.
Synthesis of compounds 35–43. Reagents and conditions: i) Aryl-amine, NaBH(OAc)3, AcOH, DCE, rt; ii) aryl acetic acid, T3P, pyridine, DMF, rt or 60 °C; iv) 2-fluoropyridine-3-boronic acid, Pd(dppf)Cl2.DCM, K2CO3, dioxane:H2O, 100 °C; v) aq. HCl, dioxane, 80 °C; vi) 1-THP-4-Bpin pyrazole, Pd(dppf)Cl2.DCM, K2CO3, dioxane:H2O, 100 °C; vii) 4M HCl, Dioxane, 60 °C; viii) B2pin2, Pd(dppf)Cl2.DCM, KOAc, dioxane, 100 °C; ix) 4-bromo-1-trityl-imidazole, Pd(dppf)Cl2.DCM, K2CO3, dioxane:H2O, 100 °C; x) AcOH, MeOH, rt; xi) 1-THP-4-Bpin pyrazole, S/XPhos Pd G2, K2CO3, 1-BuOH, 100 °C; xii) 50, cyanuric fluoride, pyridine, DCM, rt then 66 or 67, Et3N, THF 60 °C.