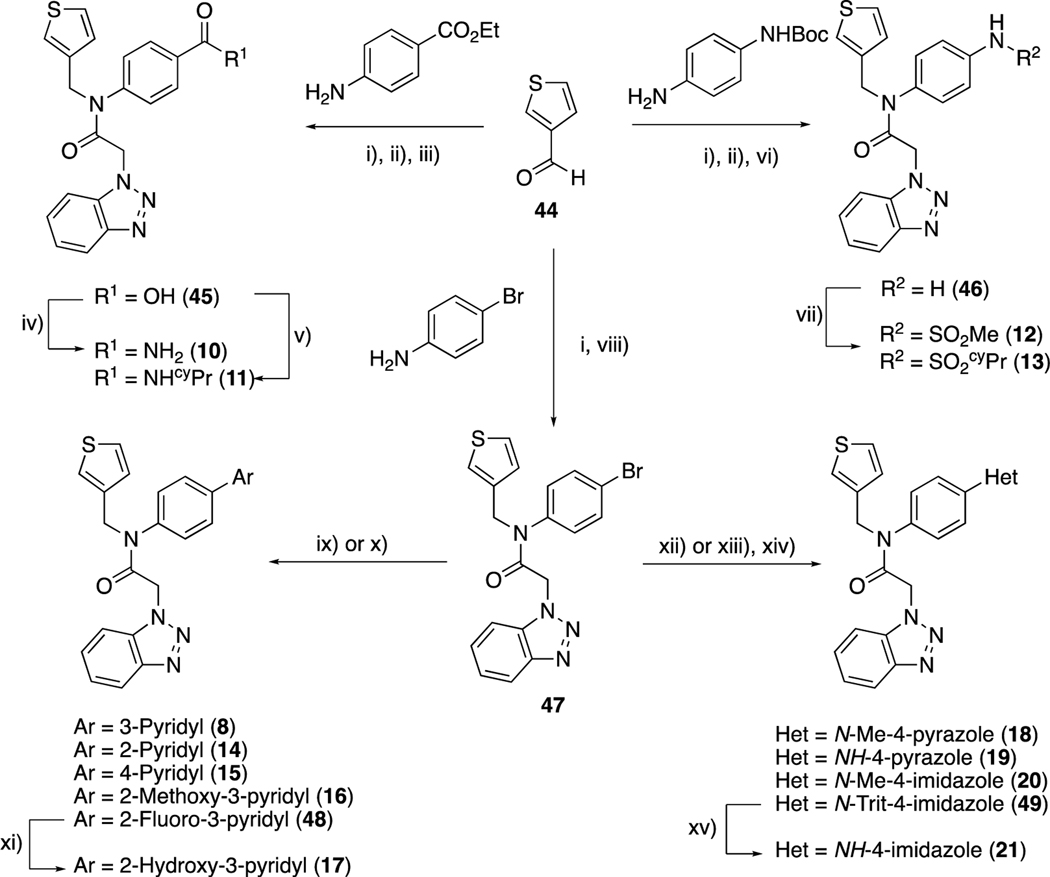
Scheme 1.
Synthesis of compounds 8–21, from thiophene-3-carbaldehyde, 44. Reagents and conditions: i) NaBH(OAc)3, AcOH, DCE, rt; ii) benzotriazole-1-acetic acid (50), T3P, EtOAc, rt; iii) LiOH, THF, rt; iv) NH4Cl, EDC, HOBt, Et3N, THF, rt; v) cyclopropylamine, COMU, DIPEA, DCM, rt; vi) TFA, DCM, rt; vii) R-SO2Cl, Et3N, DCM, rt; viii) 50, HATU, Et3N, DCM, rt; ix) ArB(OH)2, Pd(OAc)2, dppf, CuCl, Cs2CO3, DMF, 100 °C; x) Ar-B(OH)2, Pd(PPh3)4, THF:H2O, 100 °C; xi) aq. HCl, dioxane, 80 °C; xii) Ar-Bpin, Pd(dppf)Cl2.DCM, K2CO3, dioxane, 100 °C; xiii) B2pin2, Pd(dppf)Cl2.DCM, KOAc, dioxane, 80 °C; xiv) 4-bromo-1-trityl-imidazole, Pd(dppf)Cl2.DCM, K2CO3, dioxane, 100 °C; xv) AcOH, MeOH, 65 °C.