ABSTRACT
Background
Functional movement disorders (FMD) are associated with considerable morbidity and impairment of quality of life. Specialized treatment is scarce and data on efficacy of different therapies are limited.
Objective
To evaluate a multi‐modal inpatient treatment program for patients with FMD.
Methods
Thirty‐one patients with FMD were analyzed before (t1) and after multi‐modal inpatient treatment (t2) by a blinded video rating using the Psychogenic Movement Disorder Rating Scale (PMDRS), the simplified Functional Movement Disorder Rating Scale (S‐FMDRS), and the Clinical Global Impression Scale of Severity (CGI‐S), as well as patients' self‐rating. In 23 out of 31 patients a 5 months follow‐up investigation was performed (t3). Wilcoxon signed‐rank test and Friedman test were used for rating scale and self‐rating comparisons over time. Spearman correlation was used for correlation of symptom improvement and clinical characteristics.
Results
Video rating revealed significant reduction of scores after therapy (median PMDRS t1 = 24, t2 = 8, P = 0.0006; S‐FMDRS t1 = 11, t2 = 4, P = 0.008; CGI‐S t1 = 4, t2 = 3, P = 0.000136) with sustained score decrease in follow‐up evaluations (PMDRS t1 = 31, t2 = 8, t3 = 7, P = 0.000032; S‐FMDRS t1 = 12, t2 = 4, t3 = 3, P = 0.000888; CGI‐S t1 = 4, t2 = 3, t3 = 3, P = 0.000032). Patients reported a stable reduction of symptoms in the self‐rating (CGI‐S t1 = 5, t2 = 4, t3 = 4, P = 0.016). Age correlated with treatment response with older patients showing better improvement, but disease duration did not correlate with outcome. Patients who suffered from physical trauma, sexual or physical abuse had smaller score reductions.
Conclusion
Blinded video and self‐rating assessment showed significant score reduction in patients with FMD after an individualized interdisciplinary inpatient intervention.
Keywords: functional movement disorders, multi‐disciplinary therapy, physiotherapy, cognitive behavioral therapy, video rating
Functional movement disorders (FMD) are common both in general neurology outpatient clinics and specialized referral centers1 and are associated with considerable morbidity, impairment of quality of life, and burden for the health care system.2, 3 They are defined by certain characteristic clinical features including symptom attenuation by distraction, entrainment, or unusual movement patterns, allowing one to make a “positive diagnosis,” i.e. FMD is not a diagnosis of exclusion.4 However, their resemblance with other movement disorders in the context of defined neurological diseases can be challenging diagnostically and sometimes require more in‐depth neurological investigations. Underscoring the relevance of FMD, diagnostic criteria have been established5 and several assessment tools are available.6 However, despite FMD being frequent and often debilitating, therapeutic strategies are unclear, centers offering specialized treatment are scarce and data on the efficacy of different therapies are limited.6 In particular, the respective roles of neurologists, psychiatrists, and other health care providers are much debated. Several studies7, 8 assessing the efficacy of physiotherapy (motor retraining, walking, physical exercise9) and cognitive behavioral psychotherapy (CBT)10, 11 have shown promising results in the treatment of FMD. However, the duration of interventions and therapeutic settings varied considerably between these studies and there are still knowledge gaps with respect to prognostic factors and long‐term outcome of different therapeutic strategies. Against this background and because there is some evidence that FMDs reflect maladaptive integration of psychological and physical functions12, 13 one of the open questions is whether patients would benefit from a combination of psychological and physical treatment in a multi‐disciplinary team as has recently been suggested.14, 15, 16, 17, 18, 19
To this end, we here report results of a rater‐blinded retrospective evaluation of a multi‐disciplinary inpatient intervention including neurologists, psychologists, and physiotherapists in a group of 31 patients with FMD from a large center for medical treatment and rehabilitation for patients with Parkinson's disease and other movement disorders.
Methods
Patients
We performed a retrospective review of charts of 58 consecutive patients with FMD (10/2017 to 02/2020) treated at the Movement Disorders Clinic in Beelitz‐Heilstätten, Germany. Patients were referred to our center by neurologists or GPs for the assessment of an unclear movement disorder or treatment of an already diagnosed FMD, without any restrictions concerning symptom severity, age, or chronicity. However, patients with current substance abuse or unwillingness to undergo inpatient treatment were not admitted.
Inclusion criteria for the retrospective analysis were (1) definite diagnosis of a FMD;20 (2) video documentation of relevant symptoms; and (3) completed self‐rating of patients' symptom severity using the Clinical Global Impression Severity scale (CGI‐S) before and following the intervention. Twenty‐seven patients had to be excluded from analysis due to comorbidity with a relevant neurological disease (n = 3), premature discontinuation of treatment (n = 10), or lack of video documentation (n = 14). Data of 31 patients (14 male, median age 47 years, range: 19–64 years) were available for analysis. The median duration of FMD at admission was 36 months (range 3–130 months).
Before admission to our specific treatment program for FMD, most of the patients had received different types of psychotherapeutic interventions (in‐patient in 15, out‐patient in 21) or rehabilitation (n = 19, in‐patient in all cases). FMD was the reason for referral to these interventions in all cases but, to our knowledge, treatment programs were not specifically designed for FMD. Only six patients were working before admission, 10 were on sick‐leave (three of these had additional pending applications for retirement on medical grounds), and 11 were retired (eight of these had limited retirements on medical grounds) (Table 1). There was a high prevalence of depression/anxiety (n = 27), pain (n = 22), episodes of dissociation (n = 14), post‐traumatic stress disorder (n = 12), trauma (physical n = 10, psychological n = 22), and abuse (physical n = 7, sexual n = 3) in our sample (Table 2).
TABLE 1.
Demographic information of participating patients
| Sex | n (%) |
| Female | 17 (55) |
| Male | 14 (45) |
| Age, median (range) | 47 (19–64) |
| Family status | |
| Single | 12 (39) |
| Relationship | 19 (61) |
| Employment status | |
| Employed | 6 (20) |
| Unable to work | 10 (33) |
| Retired (temporary) | 8 (27) |
| Retired (unlimited) | 3 (10) |
| Application for retirement | 3 (10) |
TABLE 2.
Clinical characteristics of participating patients
| Duration of disease in months, median (range) | 36 (3–130) |
|---|---|
| Clinical symptoms | n (%) |
| Gait and balance disturbance | 21 (68) |
| Tremor | 17 (55) |
| Myoclonus | 4 (13) |
| Chorea | 6 (19) |
| Dystonia | 6 (19) |
| Paresis | 8 (26) |
| Speech disturbance | 5 (16) |
| Tics | 1 (3) |
| Others | 8 (26) |
| Multiple motor symptoms >2 | 25 (81) |
| Precipitating factors | n (%) |
| Physical trauma | 10 (32) |
| Psychological trauma | 22 (71) |
| History of depression/ anxiety | 27 (87) |
| Physical abuse | 7 (22) |
| Sexual abuse | 3 (10) |
| Post‐traumatic stress disorder (PTSD) | 12 (39) |
| Chronic pain | 22 (71) |
| Additional information | n (%) |
| Dissociative states | 14 (45) |
| Initial contact to an emergency room | 5 (16) |
Clinical Assessments
Assessment of symptom severity and treatment outcome was based on a blinded video rating. In our study, we aimed to investigate potential treatment efficacy in a standardized and objective way by validated outcome measures. We, therefore, followed the recommendation of the Functional Neurological Disorders (FND)‐Core Outcome Measures Group, who concluded that the simplified Functional Movement Disorder Rating Scale (S‐FMDRS),21 the Psychogenic Movement Disorder Rating Scale (PMDRS)22 and the CGI, which were used in several studies have good convergent sensitivity, high inter‐rater reliability and adequate sensitivity to detect symptom changes.6 The scales have already been validated in video‐based, blinded rater analysis. Moreover, the PMDRS and the S‐FMDRS were specifically designed for FMD. The S‐FMDRS is a modified (simplified) version of the PMDRS.21 The PMDRS itself has 10 different phenomenological categories and 13 different body locations the rater has to evaluate.22 This most likely leads to poorer rater agreement on symptom phenomenology and distribution in comparison to the S‐FMDRS. However, both scales showed similar high inter‐rater reliability and good sensitivity to detect symptom changes.6
Patients' symptoms were recorded by the leading physician (TS) to document movement abnormalities present at every time‐point. Because of variable phenomenology, video recordings were individualized. Blinded rating of videos was performed by a movement disorder specialist (AW). Videos were presented in random order to the blinded rater, who did not have personal contact with any of the patients and was unaware at which treatment state the individual video was taken.
In addition, patients performed a self‐rating of symptoms using the CGI‐S.
Video and self‐ratings were performed by all 31 patients before (t1) and immediately after the intervention (t2). In addition, 23 of the 31 patients participated in a follow‐up investigation that was scheduled on a voluntary basis and timed according to individual needs (t3, median = 5 months, range = 1–20 months).
Psychiatric diagnoses were based on psychopathological findings and medical history according to the diagnostic criteria of the 10th revision of the International Classification of Disease (ICD‐10).
Intervention
We used an established individualized, multi‐disciplinary treatment program. The multi‐disciplinary team comprised neurologists, psychotherapists (CBT), physiotherapists, speech therapists, music therapists, occupational therapists, and nurses. The leading physician in charge of the program (TS) is a neurologist, psychiatrist, and psychotherapist. All team members were trained in communication and interaction with FMD patients. An accepting rather than goal‐demanding attitude and an appreciative and trusting relationship towards the patient were encouraged.
Integrative networking between disciplines was supported by weekly team meetings and frequent informal briefings between team members. Special emphasis was given to coordinate therapeutic strategies and aims and to cope with emotional reactions evoked in the therapeutic process.
The program had the following main objectives: 1. to develop a consistent and easily comprehensible disease concept; 2. to improve body perception; 3. to re‐train physiological movement patterns; 4. to re‐integrate traumatizing or emotionally overwhelming experiences.
Psychotherapeutic interventions included methods of CBT and trauma therapeutic interventions. In all cases with trauma, a narrative approach to reported trauma content was conducted.
When judged as appropriate by the psychologist, elements of IRRT (Imagery Reprocessing and Rescripting Therapy)23 were additionally used to work imaginatively with the trauma experiences.
The initial focus of physiotherapy was the enhancement of body awareness. Subsequently, physiological movement sequences were exercised in the course of motor retraining. Other therapies listed above followed the principles of physiotherapy (Tables S1 and S2).
The duration of in‐patient treatment (median = 21 days, range = 10–35 days) and therapeutic plans were tailored to the individual needs of patients as perceived by the integrated team. The median duration is 50% longer than standard multi‐disciplinary in‐patient treatment, e.g., for Parkinson's disease in Germany. In general, patients with severe or chronic courses required a longer treatment period.
The program was comprised of 10–15 individual and group sessions per week with flexible involvement of different professional disciplines including 2–3 psychotherapeutic and 3–4 individual physiotherapy units. The duration of sessions ranged from 20–60 minutes according to the content and goal of the interventions.
Statistical Analysis
As our data were not normally distributed we used non‐parametric approaches throughout.
Wilcoxon signed‐rank tests were used for intraindividual comparisons of blinded video rating scores (PMDRS, S‐FMDRS, and CGI‐S) and patients self‐rated CGI‐S before (t1) and immediately after the end of the inpatient program (t2) in 31 patients.
To analyze potential symptom changes after the discharge from the inpatient treatment, we compared video rating and self‐rating results of 23 patients at three time points (pre‐ t1, post‐intervention t2, and follow‐up t3) using the Friedman ANOVA.
To correct for multiple comparisons (the factor eight, e.g., for the two or three time points and the four rating scales/self‐questionnaires was used) Bonferroni correction was used.
Correlation of the continuous variables: symptom improvement (=clinical scores differences between t1 and t2 of the video rating and self‐rating), age at examination, and disease duration in months, was estimated using Spearman correlation. To analyze for potential influences of categorical variables such as demographic and clinical features (Tables 1 and 2) on symptom improvement (=clinical scores differences between t1 and t2 of the video rating and self‐rating) Mann Whitney tests were used. Correlations and Mann Whitney tests were done at a descriptive level only without interpretation of statistical significance and correction for multiple comparisons.
Results
On the basis of the blinded video assessment, the intervention reduced symptoms significantly in all three scales (median PMDRS t1 = 24 (range: 6–74), t2 = 8 (range: 0–48), P = 0.000078 uncorrected, P = 0.0006 corrected; median S‐FMDRS t1 = 11 (range: 2–24), t2 = 4 (range: 0–29), P = 0.001 uncorrected, P = 0.008 corrected; median CGI‐S t1 = 4 (range: 3–6), t2 = 3 (range: 1–5), P < 0.000068 uncorrected, P = 0.000136 uncorrected) (Fig 1A,B).
FIG. 1.
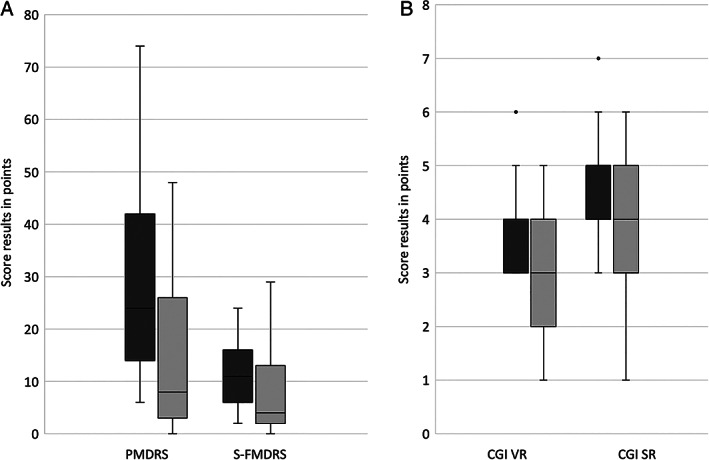
Box plots of the rating results of 31 patients using (A) the PMDRS (=psychogenic movement disorders rating scale) and S‐FMDRS (=simplified functional movement disorders rating scale) of the video rating, and (B) the CGI (=clinical global impression scale) of the video rating (VR) and the CGI of patients' self‐rating (SR). Dark gray bars = before therapy (t1), light gray bars = immediately after therapy (t2); black line indicates median, boxes the quartiles, whiskers the range, and circles the outlier.
Likewise, CGI‐S of the self‐rating was significantly improved (median t1 = 5 (range: 3–7), t2 = 4 (range: 1–6), P = 0.005 uncorrected, P = 0.004 corrected) (Fig 1B).
Comparison of the severity scores in the 23 patients with a follow‐up investigation showed a significant reduction of the symptom severity after intervention and in the follow‐up (median PMDRS t1 = 31 (range: 7–74), t2 = 8 (range: 0–48), t3 = 7 (range: 0–45), P = 0.000004 uncorrected, P = 0.000032 corrected; median S‐FMDRS t1 = 12 (range: 3–22), t2 = 4 (range: 0–29), t3 = 3 (range: 0–23), P = 0.000111 uncorrected, P = 0.000888 corrected; median CGI‐S t1 = 4 (range: 3–6), t2 = 3 (range: 1–5), t3 = 3 (range: 1–4), P = 0.000004 uncorrected, P = 0.000032 corrected) (Fig 2A,B). Scores differed between t1 and t2, as well as between t1 and t3, but not between t2 and t3, suggesting a stable improvement of symptoms after a median of 5 months post‐intervention.
FIG. 2.
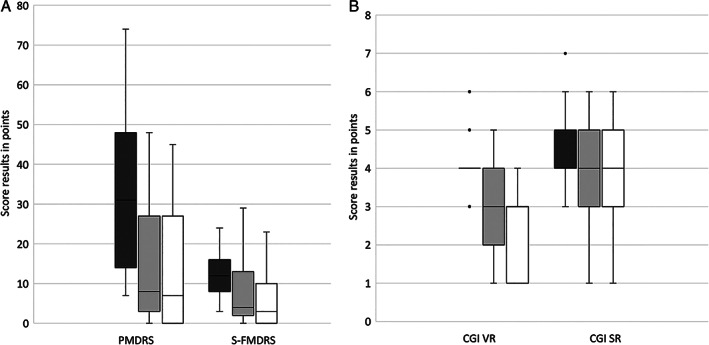
Box plots of the rating results of 23 patients using (A) the PMDRS (=psychogenic movement disorders rating scale) and S‐FMDRS (=simplified functional movement disorders rating scale) of the video rating, and (B) the CGI (=clinical global impression scale) of the video rating (VR) and the CGI of patients' self‐rating (SR). Dark gray bars = before therapy (t1), light gray bars = immediately after therapy (t2), white bars = follow‐up (t3); black line indicate median, boxes the quartiles, whiskers the range, and circles the outlier.
Self‐rating CGI‐S by 23 patients at three time points showed a significant decline of symptoms that remained stable at outpatient follow‐up (t1 = 5 (range: 3–7), t2 = 4 (range: 1–6), t3 = 4 (range: 3–5), P = 0.002 uncorrected, P = 0.016 corrected) (Fig 2B).
There was a positive correlation between age at examination and the changes of the PMDRS (descriptive P = 0.045, r = 0.363), i.e., older patients had greater improvement e.g., higher change of scores (t1‐t2). It is possible that in view of higher baseline scores the likelihood of significant changes at t2, e.g., improvement of symptoms and reduction of PMDRS scores, was higher in this sub‐sample. There was no correlation between age at examination and the other scores used. Duration of symptoms at baseline did not correlate with outcome e.g. the changes of any of the rating scales used.
Patients who experienced a physical trauma showed smaller changes in the PMDRS (physical trauma present median PMDRS t1 = 14, t2 = 10 compared to physical trauma absent median PMDRS t1 = 28, t2 = 8; descriptive P = 0.005) and S‐FMDRS score compared to those without a physical trauma (physical trauma present median S‐FMDRS t1 = 8, t2 = 4 compared to physical trauma absent median S‐FMDRS t1 = 11, t2 = 4; descriptive P = 0.018). GCI‐S of the video and self‐rating did not differ between patients with and without trauma (Table S3).
Patients who had been physically or sexually abused had smaller changes in the S‐FMDRS (physical abuse present: median S‐FMDRS t1 = 10, t2 = 4 compared to physical abuse absent: median S‐FMDRS t1 = 9, t2 = 3; descriptive P = 0.034; sexual abuse present: median S‐FMDRS t1 = 13, t2 = 16 compared to sexual abuse absent median: S‐FMDRS t1 = 9, t2 = 4; descriptive P = 0.015).
Patients who received treatment (inpatient rehabilitation, outpatient, or inpatient psychotherapy) before participating in our treatment program did not show better treatment responses, e.g., higher reduction of scores in video rating and self‐rating. There was no correlation between the factors inpatient rehabilitation, inpatient or outpatient psychotherapy and the changes of PMDRS, S‐FMDRS, or CGI‐S. Also other demographic and clinical variables did not affect scores (Table S3).
In an informal interview at discharge, 18 of 31 patients spontaneously expressed that, in their view, friendliness, appreciation, acceptance, and active listening were key factors for success of their treatment. In addition, the importance of the multi‐disciplinary approach was emphasized.
Discussion
The main finding of the present study with independent blinded video assessment is that patients with FMD who completed the individualized comprehensive interdisciplinary inpatient intervention combining physiotherapy and psychological measures showed significantly reduced motor symptoms. Interestingly, and in correspondence with other multi‐disciplinary treatment studies,16, 18 we did not find a correlation between symptom duration and score results, indicating that prognosis might not necessarily be poor in patients with chronic FMD, who receive multi‐disciplinary treatment.
According to Ricciardi and Edward,24 there are probably different routes to the development and maintenance of FMD so that individualized approaches appear warranted. Consensus recommendations have emphasized the importance of physiotherapy for the treatment of FMD25 based on prospective studies reporting its usefulness both on an inpatient26, 27, 28 and outpatient basis.7, 15 The rationale for physiotherapy has to be viewed against the background that there is some evidence that FMD are involuntary but learned habitual movement patterns driven by abnormal self‐directed attention.29 Therefore, one aim of physiotherapy is motor‐retraining directed towards the goal rather than sub‐elements or kinematics of movement to divert self‐focused attention and to prevent patients from cognitively controlling their movements.25, 29 In the present study, we slightly adapted this concept (Table S2). Thus, physiotherapy first focused on improving the perception of the affected body region and the whole body to access and integrate segregated functions, which was then followed by motor retraining. Feedback from the patient during mindfulness exercises and motor‐retraining was used to foster awareness and a sense of agency. The latter has been shown to be impaired in patients with FMD.12 During the training, the autonomy of the patient was respected and motor challenges were individually scaled.
In keeping with a recently proposed model of integrated and multi‐disciplinary care for patients with FMD,14 we complemented physiotherapy with psychological measures. In a recent study applying 12 weeks of CBT, the effectiveness in reducing the severity of functional tremor was shown.11
There is general agreement that communication of the diagnosis and providing of a bio‐psychological model, within which to understand the physical symptoms, is critical to establish a therapeutic relationship with patients with FMD.4 Our integrated team approach was based on the concept of a “motive oriented therapeutic relationship” (MOTHER).30, 31 This concept postulates four basic human needs, i.e., attachment, self‐esteem, security, and pleasure.31 The therapist is oriented towards the patient's perceived need for relationship, e.g., beyond an appreciative attitude, the therapist responds to the patient's basic relational concerns. These are assumed to be learning‐ and experience‐based, both life‐historical and short‐term in origin. This also includes the patient's previous experiences within the medical system. Moreover, methods of CBT and emotionally focused techniques were applied.32 We first addressed and validated the beliefs and concerns of the patients. In close cooperation with the patients, a bio‐psychological concept and an understanding of the genesis of the disease were developed. The conscious perception of patients' emotions was encouraged and supported (Table S1).
We opted for a multi‐professional team suitable for intervening in parallel at different functional levels. Achievements in different treatment modalities probably were complementary and might have had a multiplier effect. Beyond logistic coordination, the integrated network approach supports the acquisition of therapeutic skills. Providers learn to implement techniques across different disciplines, e.g., combining physiotherapy and psycho‐education.
Our general setting, i.e., a specialized clinic for patients with movement disorders including patients with defined neurological diseases, e.g., Parkinson's disease, also might have influenced the results of the intervention. Many patients with FMD have had made the experience of being poorly understood or even stigmatized in the standard health care system including neurological services and being referred to psychiatric units without further explanations or concepts where they are then told that psychiatrically there is “nothing wrong” with them.33 More often than not, patients with FMD felt left alone. In fact, patients in our sample have had numerous contacts with the health care system (24 of 31 were pre‐treated including out‐ or inpatient psychotherapy, or rehabilitation) without substantial improvement of their symptoms. Because patients with FMD often attribute their symptoms to organic disturbances, as did our patients, it appears suitable and helpful to treat these patients in movement disorder clinics treating patients with both organic diseases and FMD to avoid stigmatization or marginalization.
Regarding co‐morbidities and prognosis, 23 of 31 patients had signs of traumatic experiences in the past. Patients with a history of physical trauma and sexual or physical abuse had less improvement of symptoms. This is in line with previous observations that a history of trauma is more frequent in FMD compared to patients with “organic” movement disorders.34 By using trauma therapeutic methods (narrative, or techniques from Imagery Rescripting and Processing Therapy)35 we aimed to integrate traumatically unprocessed triggering experiences into conscious biographical memory content.
In a previous review, Gelauff and colleagues found four studies that showed a better clinical outcome in younger patients and seven studies where age did not affect the outcome.36 In contrast, older age was correlated with a better outcome in our study.
A short duration of symptoms has been suggested as a positive prognostic features.36 However, in accordance with others,15 we did not find a correlation between disease duration and changes of clinical rating scales. In fact, several patients with long‐standing symptoms and a long history of previous somatic and psychotherapeutic interventions remitted. However, our correlations were carried out for descriptive purposes only and power was probably too low. Therefore, these descriptive data have to be interpreted cautiously.
There are other limitations. Our design was retrospective. Although video recordings were evaluated in a blinded fashion, we did not use a standardized video protocol, which might have limited the validity of our data. Filming was performed to capture all symptoms, but we cannot exclude that subtle abnormalities were missed. Our analysis refers only to the 31 patients who finished the inpatient therapy program. Also, the follow‐up investigation was available only in about two‐thirds of patients and intervals between discharge and the last follow‐up were variable so that these data also need to be interpreted with caution. Despite these limitations, the data from follow‐up assessments still suggest that sustained improvement can be achieved in a large proportion of patients.
A longstanding experience of all our team members in the diagnosis and treatment of FMD and the focus on a trusting, motive‐oriented relationships may explain, at least in part, why patients who had undergone previous treatments benefited from our FMD treatment program.
At present, the heterogeneity of therapeutic interventions, study designs, and patient samples do not allow direct comparison of our and other models of care for FMD. Yet, the accumulated empirical knowledge allows for formulating testable hypotheses for the treatment of FMD. Prospective controlled protocols are warranted to compare the efficacy and cost‐effectiveness of different treatment concepts, e.g., single profession versus multi‐disciplinary treatment and to better understand prognostic factors such as co‐morbidity, age, and chronicity of FMD.
To summarize, in this retrospective study with independent blinded assessment, individualized comprehensive interdisciplinary inpatients intervention combining physiotherapy and psychological measures significantly reduced symptoms in patients with FMD.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the first draft, B. Review and Critique.
T.S.: 1A, 1B, 1C, 2C, 3A, 3B
G.E.: 1A, 2C, 3B
H.O.: 1C, 2C, 3B
A.S.: 1C, 2C, 3B
I.R.K.: 1C, 2A, 2C, 3B
T.B.: 1A, 2A, 2C, 3B
A.M.: 1A, 2C, 3B
A.W.: 1A, 1C, 2A, 2B, 3B
Disclosures
Ethical Compliance Statement
Approval of the local ethic committee of the Movement Disorders Clinic Beelitz, Germany was obtained for the retrospective review of charts and videos. Informed consent of all patients was obtained prior to inclusion of the study. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest
This work was support by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG, FOR 2698). There is no conflict of interest.
Financial Disclosures for the Previous 12 Months
Tamara Schmidt received honoraria for a lecture in 12/20 from Licher MT GmbH. Georg Ebersbach received funding for consultancies from AbbVie Pharma, BIAL Pharma, Biogen GmbH, Desitin Pharma, STADA Pharma, Neuroderm Inc. He is at the advisory boards of AbbVie Pharma, BIAL Pharma, Biogen GmbH, Desitin Pharma, STADA Pharma, Neuroderm Inc. He receives honoraria from Speakers Honorary: AbbVie Pharma, BIAL Pharma, Britannia Pharma, Desitin Pharma, Licher GmbH, UCB Pharma, Zambon Pharma. He has royalties on Kohlhammer Verlag, Thieme Verlag. Henriette Oelsner and Anette Sprock do not have financial disclosures. Inke R. König received grants from the German Research Foundation, BMBF, German Cancer Aid. Tobias Bäumer iis at the advisory board of Ipsen Pharma, Allergan, Merz Pharmaceuticals. He received honoraria from Ipsen Pharma, Allergan, Merz Pharmaceuticals. He receives a grant from the Research Group, DFG FOR 2698. Alexander Münchau reports consultancies for Desitin, Merz Pharmaceuticals, Admedicum. He is at the advisory board of German Tourette syndrome Association; Alliance of patients with chronic rare diseases. He receives honoraria of Pharm Allergan, Ipsen, Merz Pharmaceuticals, Actelion, GlaxoSmithKline, Desitin, Teva, Takeda. He receives grants from Possehl‐Stiftung (Lübeck, Germany), Margot und Jürgen Wessel Stiftung (Lübeck, Germany), Tourette Syndrome Association (Germany), Interessenverband Tourette Syndrom (Germany), CHDI, Damp‐Stiftung (Kiel, Germany); Deutsche Forschungsgemeinschaft (DFG): projects 1692/3–1, 4–1, SFB 936, and FOR 2698 (project numbers 396,914,663, 396,577,296, 396,474,989); European Reference Network—Rare Neurological Diseases (ERN—RND; Project ID No 739510). He receives royalties for the book Neurogenetics (Oxford University Press). Anne Weissbach receives grants from the Else Kröner‐Fresenius grant (EKFS, 2018_A55), German Research Foundation (DFG, WE5919/2–1) and Edmond J. Safra Fellowship in Movement Disorders from the Michael J. Fox foundation.
Supporting information
Table S1. Treatment goals and characteristics of the psychotherapy/ behavioral therapy intervention.
Table S2. Characteristics of physiotherapy intervention.
Table S3. Video rating and self‐rating score results of patients with functional movement disorders with different demographic and clinical characteristics.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1.Stone J, Carson A, Duncan R, et al. Who is referred to neurology clinics?—the diagnoses made in 3781 new patients. Clin Neurol Neurosurg 2010;112(9):747–751. [DOI] [PubMed] [Google Scholar]
- 2.Carson AJ, Ringbauer B, Stone J, McKenzie L, Warlow C, Sharpe M. Do medically unexplained symptoms matter? A prospective cohort study of 300 new referrals to neurology outpatient clinics. J Neurol Neurosurg Psychiatry 2000;68(2):207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barsky AJ, Orav EJ, Bates DW. Somatization increases medical utilization and costs independent of psychiatric and medical comorbidity. Arch Gen Psychiatry 2005;62(8):903–910. [DOI] [PubMed] [Google Scholar]
- 4.Edwards MJ, Bhatia KP. Functional (psychogenic) movement disorders: merging mind and brain. Lancet Neurol 2012;11(3):250–260. [DOI] [PubMed] [Google Scholar]
- 5.Gasca‐Salas C, Lang AE. Neurologic diagnostic criteria for functional neurologic disorders. Handb Clin Neurol 2016;139:193–212. [DOI] [PubMed] [Google Scholar]
- 6.Pick S, Anderson DG, Asadi‐Pooya AA, et al. Outcome measurement in functional neurological disorder: a systematic review and recommendations. J Neurol Neurosurg Psychiatry 2020;91(6):638–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nielsen G, Ricciardi L, Demartini B, Hunter R, Joyce E, Edwards MJ. Outcomes of a 5‐day physiotherapy programme for functional (psychogenic) motor disorders. J Neurol 2015;262(3):674–681. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen G, Buszewicz M, Stevenson F, et al. Randomised feasibility study of physiotherapy for patients with functional motor symptoms. J Neurol Neurosurg Psychiatry 2017;88(6):484–490. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen G, Stone J, Buszewicz M, et al. Physio4FMD: protocol for a multicentre randomised controlled trial of specialist physiotherapy for functional motor disorder. BMC Neurol 2019;19(1):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dallocchio C, Tinazzi M, Bombieri F, Arno N, Erro R. Cognitive Behavioural therapy and adjunctive physical activity for functional movement disorders (conversion disorder): a pilot, single‐blinded, randomized study. Psychother Psychosom 2016;85(6):381–383. [DOI] [PubMed] [Google Scholar]
- 11.Espay AJ, Ries S, Maloney T, et al. Clinical and neural responses to cognitive behavioral therapy for functional tremor. Neurology 2019;93(19):e1787–e1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parees I, Brown H, Nuruki A, et al. Loss of sensory attenuation in patients with functional (psychogenic) movement disorders. Brain 2014;137(Pt 11):2916–2921. [DOI] [PubMed] [Google Scholar]
- 13.Sadnicka A, Daum C, Meppelink AM, Manohar S, Edwards M. Reduced drift rate: a biomarker of impaired information processing in functional movement disorders. Brain 2020;143(2):674–683. [DOI] [PubMed] [Google Scholar]
- 14.Lidstone SC, MacGillivray L, Lang AE. Integrated therapy for functional movement disorders: time for a change. Mov Disord Clin Pract 2020;7(2):169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacob AE, Kaelin DL, Roach AR, Ziegler CH, LaFaver K. Motor retraining (MoRe) for functional movement disorders: outcomes from a 1‐week multidisciplinary rehabilitation program. PM R 2018;10(11):1164–1172. [DOI] [PubMed] [Google Scholar]
- 16.McCormack R, Moriarty J, Mellers JD, et al. Specialist inpatient treatment for severe motor conversion disorder: a retrospective comparative study. J Neurol Neurosurg Psychiatry 2014;85(8):895–900. [DOI] [PubMed] [Google Scholar]
- 17.Demartini B, Batla A, Petrochilos P, Fisher L, Edwards MJ, Joyce E. Multidisciplinary treatment for functional neurological symptoms: a prospective study. J Neurol 2014;261(12):2370–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saifee TA, Kassavetis P, Parees I, et al. Inpatient treatment of functional motor symptoms: a long‐term follow‐up study. J Neurol 2012;259(9):1958–1963. [DOI] [PubMed] [Google Scholar]
- 19.Moene FC, Spinhoven P, Hoogduin KA, van Dyck R. A randomised controlled clinical trial on the additional effect of hypnosis in a comprehensive treatment programme for in‐patients with conversion disorder of the motor type. Psychother Psychosom 2002;71(2):66–76. [DOI] [PubMed] [Google Scholar]
- 20.Espay AJ, Lang AE. Phenotype‐specific diagnosis of functional (psychogenic) movement disorders. Curr Neurol Neurosci Rep 2015;15(6):32. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen G, Ricciardi L, Meppelink AM, Holt K, Teodoro T, Edwards M. A simplified version of the psychogenic movement disorders rating scale: the simplified functional movement disorders rating scale (S‐FMDRS). Mov Disord Clin Pract. 2017;4(5):710–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinson VK, Cubo E, Comella CL, Goetz CG, Leurgans S. Rating scale for psychogenic movement disorders: scale development and clinimetric testing. Mov Disord 2005;20(12):1592–1597. [DOI] [PubMed] [Google Scholar]
- 23.Smucker MR. Imagery rescripting and reprocessing therapy. Encyclopedia of Cognitive Behavior Therapy. Boston, MA: Springer; 2005:226–229. [Google Scholar]
- 24.Ricciardi L, Edwards MJ. Treatment of functional (psychogenic) movement disorders. Neurotherapeutics 2014;11(1):201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen G, Stone J, Matthews A, et al. Physiotherapy for functional motor disorders: a consensus recommendation. J Neurol Neurosurg Psychiatry 2015;86(10):1113–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Czarnecki K, Thompson JM, Seime R, Geda YE, Duffy JR, Ahlskog JE. Functional movement disorders: successful treatment with a physical therapy rehabilitation protocol. Parkinsonism Relat Disord 2012;18(3):247–251. [DOI] [PubMed] [Google Scholar]
- 27.Jordbru AA, Smedstad LM, Klungsoyr O, Martinsen EW. Psychogenic gait disorder: a randomized controlled trial of physical rehabilitation with one‐year follow‐up. J Rehabil Med 2014;46(2):181–187. [DOI] [PubMed] [Google Scholar]
- 28.Dallocchio C, Arbasino C, Klersy C, Marchioni E. The effects of physical activity on psychogenic movement disorders. Mov Disord 2010;25(4):421–425. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen G. Physical treatment of functional neurologic disorders. Handb Clin Neurol 2016;139:555–569. [DOI] [PubMed] [Google Scholar]
- 30.Caspar F‐M. Motivorientierte Beziehungsgestaltung. Konzept, Voraussetzungen bei den Patienten und Auswirkungen auf Prozess und Ergebnisse. Handbuch der therapeutischen Beziehung. Allgemeiner Teil, Bd. 1. Tübingen: DGVT‐Verlag; 2008:527–558.
- 31.Grawe K. Psychologische Therapie. Verlag für Psychologie: Hogrefe; 1998. [Google Scholar]
- 32.Greenberg LS. Emotion–focused therapy. Clin Psychol Psychother 2004;11(1):3–16. [DOI] [PubMed] [Google Scholar]
- 33.Stone J. The bare essentials: functional symptoms in neurology. Pract Neurol 2009;9(3):179–189. [DOI] [PubMed] [Google Scholar]
- 34.Kranick S, Ekanayake V, Martinez V, Ameli R, Hallett M, Voon V. Psychopathology and psychogenic movement disorders. Mov Disord 2011;26(10):1844–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmucker M, Köster R. Praxishandbuch IRRT: Imagery Rescripting & Reprocessing Therapy bei Traumafolgestörungen, Angst, Depression und Trauer. Vol 269. Stuttgart, Germany: Klett‐Cotta; 2014. [Google Scholar]
- 36.Gelauff J, Stone J, Edwards M, Carson A. The prognosis of functional (psychogenic) motor symptoms: a systematic review. J Neurol Neurosurg Psychiatry 2014;85(2):220–226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Treatment goals and characteristics of the psychotherapy/ behavioral therapy intervention.
Table S2. Characteristics of physiotherapy intervention.
Table S3. Video rating and self‐rating score results of patients with functional movement disorders with different demographic and clinical characteristics.


