Abstract
Laser refractive surgery is one of the most commonly performed procedures worldwide. In laser refractive surgery, Femtosecond Laser in Situ Keratomileusis and Refractive Lenticule Extraction have emerged as promising alternatives to microkeratome Laser in Situ Keratomileusis and Photorefractive Keratectomy. Following laser refractive surgery, the corneal nerves, epithelial and stromal cells release neuromediators, including neurotrophins, neuropeptides and neurotransmitters. Notably, nerve growth factor, substance P, calcitonin gene-related peptide and various cytokines are important mediators of neurogenic inflammation and corneal nerve regeneration. Alterations in neuromediator profiles and ocular surface parameters following laser refractive surgery are attributed to the surgical techniques and the severity of tissue insult induced. In this review, we will discuss the (1) Functions of neuromediators and their physiological and clinical significance; (2) Changes in the neuromediators following various laser refractive surgeries; (3) Correlation between neuromediators, ocular surface health and corneal nerve status; and (4) Future directions, including the use of neuromediators as potential biomarkers for ocular surface health following laser refractive surgery, and as adjuncts to aid in corneal regeneration after laser refractive surgery.
Key Words: cornea, corneal nerves, dry eye, femtosecond laser, laser in situ keratomileusis, neuromediator, refractive surgery, small incision lenticule extraction
Laser Refractive Surgery
Refractive error is a leading cause of reversible visual impairment worldwide (Lou et al., 2016). For the correction of refractive errors, laser refractive surgery remains a mainstay treatment in achieving spectacle independence, and is one of the most commonly performed ophthalmic surgeries globally (Kim et al., 2019). Laser refractive surgery has been established as a safe and effective procedure associated with excellent visual outcomes, improvement in the quality of life, and high patient satisfaction (Sandoval et al., 2016). The number of laser refractive surgeries has seen a burgeoning increase since its introduction in ophthalmology, with more than 16 million refractive surgeries performed worldwide (Bandeira et al., 2019).
The femtosecond laser (FSL) represents a significant milestone in ophthalmic surgery, including refractive surgery (Liu et al., 2018; Han et al., 2020), keratoplasty (Liu et al., 2019), conjunctival and cataract surgery (Fuest et al., 2017; Liu et al., 2017). The FSL utilizes ultrashort pulses of near-infrared wavelength light to make tissue incisions (Liu et al., 2015). Reducing the pulse duration to a femtosecond (10–15) level produces smaller microcavitation bubbles (Figure 1A) and shock waves, thereby reducing the degree of collateral damage (Figure 1B), which is often associated with conventional lasers such as the argon fluoride excimer laser for photoablation (Liu et al., 2015; Fuest et al., 2017). With an accuracy of 5 µm, the FSL’s ability to photodisrupt tissue with high precision is ideal for surgeries wherein precision is crucial to achieving good outcomes (Liu et al., 2016b).
Figure 1.
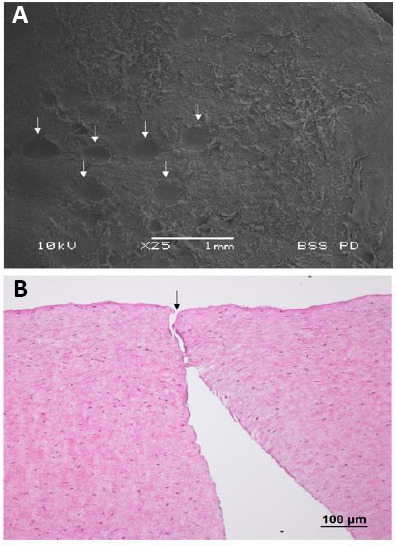
Scanning electron microscopy picture and corneal histologic section with hematoxylin and eosin staining showing the photodisruption process of femtosecond laser.
Bubbles cavities left after bubble expansion (arrows) are generated during the laser firing process, cleaving the tissue (A). The histological section shows no thermal damage, coagulative necrosis or obvious inflammatory response observed along the laser path (B; arrow). Sourced from unpublished data of the authors’ laboratory.
Femtosecond Laser in Situ Keratomileusis (FSL-LASIK) has emerged as an alternative to conventional mechanical microkeratomes for flap creation. It has been increasingly preferred due to its better precision, greater surgeon flexibility in flap characteristics, ability to produce thinner flaps, and reduced flap-related complications, attributable to the uniform flap morphology that enhances adhesion strength (Salomão and Wilson, 2010). Whilst FSL-LASIK involves two lasers, that is, the FSL for flap creation and excimer laser for stromal ablation, Refractive Lenticule Extraction (ReLEx) requires only a single FSL. Since the first study of Femtosecond Lenticule Extraction (FLEx) published in 2008 by Sekundo et al., ReLEx procedures have gained increasing popularity as a comparable, if not better, alternative to FSL-LASIK. A variant of ReLEx, Small Incision Lenticule Extraction (SMILE), is the most advanced form of ReLEx. Even though FLEx is performed without the need for an excimer laser, a corneal flap similar to the LASIK flap is created prior to lenticule extraction. On the other hand, SMILE involves a small corneal incision created by the FSL, through which the lenticule is dissected and extracted (Liu, 2016). While SMILE has been shown to have similar safety, efficacy and predictability profiles to FSL-LASIK, it provides better outcomes regarding the impact on the ocular surface, as well as with respect to corneal wound healing and inflammatory responses postoperatively (Liu et al., 2016b).
Following laser refractive surgery, the corneal nerves, epithelial and stromal cells release neuromediators that play an important role in postoperative neurogenic inflammation, wound healing and corneal nerve regeneration. In this review, we will discuss the functions of neuromediators, the changes in neuromediators following various laser refractive surgeries, and their clinical implications. The authors searched the electronic database PudMed for relevant articles relating to corneal neuromediators following laser refractive surgery. Keywords including “nerve regeneration”, “corneal healing”, “neurogenic inflammation”, “neurotrophic factors”, “nerve growth factor”, “substance P”, “calcitonin gene-related peptide”, “femtosecond laser”, “neurotrophic keratitis”, “neurotrophic keratopathy”, “ocular surface”, “corneal nerve” and “corneal biomarker” were used. Only articles published in English were used. The date of publication was restricted to the last five years as best as possible. Additional relevant articles were identified from the references of these included articles. The latest search date was September 14, 2020. After removing duplicates, we independently screened the articles to ensure fulfilment of the inclusion criteria, and subsequently assessed the full-text version of all included articles. Of 401 articles identified through database searching, 329 articles were screened, and 85 articles were eventually included in the final manuscript.
Corneal Neuromediators
Corneal neuromediators refer to the chemical substances released by corneal nerves and they include neurotrophins, neuropeptides and neurotransmitters (Al-Aqaba et al., 2019). They play a significant role not only in physiological homeostatic processes, but also in corneal wound healing following injurious stimuli (Chao et al., 2016). The release of neuromediators is vital in corneal nerve regeneration and return of normal neuronal function after refractive surgery. Moreover, the presence and levels of specific neuromediators have been shown to influence the occurrence of adverse effects following laser refractive surgery, such as postoperative dry eye, as will be discussed subsequently.
The cornea is densely innervated with an average of 351 ± 53.5 bundles per human cornea (Mansoor et al., 2020). Corneal innervation is maintained by a homeostatic and neurochemical milieu of neurotrophins, neuropeptides and neurotransmitters, a group of biologically active chemicals collectively known as neuromediators. These neuromodulating chemicals are produced by, and exert effects on a myriad of cells in the cornea, in a complex neurobiological interplay.
The cornea is innervated predominantly by sensory nerves, which confer both afferent and efferent functions, receiving touch and pain sensation, as well as producing neuromediators. Most sensory nerves produce neuropeptides and neurotrophins. Autonomic innervation of the cornea arises from sympathetic and parasympathetic fibers. The corneal sympathetic innervation, although scarce, is a source of important neurotransmitters such as catecholamines (Müller et al., 2003). On the other hand, parasympathetic nerves of the cornea produce acetylcholine. Other corneal cells apart from neurons contribute to the diversity of neuromediators in the cornea. Corneal epithelial cells are a source of acetylcholine, cholinergic synthetic and degradative enzymes, as well as neurotrophins. Neurotrophins are also derived from corneal endothelial cells and stromal cells (Lambiase et al., 2000).
Neurotrophins belong to a class of growth factors that regulate neuronal development, survival, death and plasticity (Al-Aqaba et al., 2019). They are synthesized as inactive precursors, then cleaved by extracellular proteinases to mature neurotrophins, which then activate the p75 neurotrophin receptor and tropomyosin-related tyrosine kinase receptor to affect downstream biological functions (Müller et al., 2003). Nerve growth factor (NGF) is the best-characterized neurotrophin, which is important in sustaining normal corneal nerve density and corneal sensation (Liu, 2010). NGF has garnered considerable attention for its potential in corneal nerve regeneration. Its recombinant form, cenegermin, has recently been used in patients with non-healing corneal epithelial defects in neurotrophic keratopathy (NK) to enhance the rate of corneal healing (Bonini et al., 2018). It has been postulated that NGF serves as a pleiotropic factor for an injured cornea via several mechanisms. Through in vitro studies, Aloe et al. (2015) demonstrated the role of NGF in the stimulation of corneal nerve regeneration, modulation of corneal stem cells through the induction of fibroblastic differentiation into myofibroblasts, and migration of wounded fibroblasts. NGF is also thought to play an instrumental role in the migration, colony formation and proliferation of epithelial cells, through the activation of tropomyosin receptor kinase A, a high-affinity receptor for NGF located on corneal epithelial cells (Al-Aqaba et al., 2019). Other neurotrophins, including glial-derived neurotrophic factor, brain-derived neurotrophic factor, ciliary neurotrophic factor (CNTF) and neurotrophin-3 (NT-3) have also been described in the cornea. In vitro studies of the corneal epithelium have demonstrated that glial-derived neurotrophic factor parallels the functions of NGF in cell migration, colony formation and proliferation (You et al., 2001), whereas brain-derived neurotrophic factor promotes colony formation (You et al., 2000). CNTF exerts trophic effects through the activation of corneal epithelial progenitor cells. Exogenous CNTF has been shown to accelerate healing and nerve regeneration of the wounded corneal epithelium in a mouse model (Zhou et al., 2015). Lastly, mouse corneal studies have demonstrated NT-3 as a survival factor of both sensory and sympathetic nerves, as well as a modulator of neuronal branching (Bennett et al., 2002).
Neuropeptides and neurotransmitters function as important messengers, transmitting nervous impulses from the presynaptic to the postsynaptic neuron, through the synaptic cleft. Neuropeptides are released slowly, act over an extended duration on many receptors, and exert paracrine effects aside from their neurotransmission function. Substance P (SP) and calcitonin gene-related peptide (CGRP) are the most common neuropeptides in mouse cornea (He and Bazan, 2016). SP, much like NGF, exerts a trophic effect on the rabbit corneal epithelium by modulating cell proliferation, migration and adhesion (Garcia-Hirschfeld et al., 1994). SP is constitutively expressed in tears. Its effects are important in both the maintenance of normal corneal epithelium, and healing of cornea after injurious stimuli (Al-Aqaba et al., 2019). SP exerts physiological roles in rabbit corneal homeostasis (Yamada et al., 2003), serving as an important mediator of reflex tear production, protector of corneal epithelial barrier function, and inhibitor of epithelial cell apoptosis. Interestingly, in diseased rabbit corneas, Nishida et al. (1996) established the synergistic interaction of SP with other trophic factors, such as insulin-like growth factor-1 (IGF-1), in stimulating corneal epithelial healing. Nishida et al. (2007) subsequently demonstrated the use of eye drops comprising an SP-derived peptide and IGF-1, to successfully treat epithelial defects in NK. The SP/IGF-1 complex upregulates integrin alpha-5, a fibronectin receptor, hence promoting cell adhesion to a fibronectin complex which plays a key role in epithelial cell migration and regeneration in a rabbit model (Nakamura et al., 1998). In clinical cross-sectional studies (Chao et al., 2016), tear SP levels have been shown to be positively correlated with the severity of dry eye symptoms, and negatively correlated with corneal sensitivity following LASIK. SP is also associated with corneal nerve degeneration in diabetes mellitus. Tummanapalli et al. (2019) demonstrated a significant correlation between the tear SP concentrations and corneal nerve fiber density (CNFD), as well as the Total Neuropathy Score of the severity of diabetic peripheral neuropathy. As a result, SP may serve as a useful biomarker for assessing post-laser refractive surgery dry eye and diabetic peripheral neuropathy.
CGRP is another neuropeptide expressed constitutively in tears for corneal epithelial maintenance, whose secretion increases after corneal wounding (Al-Aqaba et al., 2019). An earlier study has postulated that CGRP exerts vasoactive effects and increases blood flow in the eye after corneal epithelial injury (Uusitalo et al., 1989). CGRP has further been shown to enhance corneal re-epithelialization via the facilitation of epithelial cell migration in dog cornea, as well as cell differentiation in rabbit cornea (Garcia-Hirschfeld et al., 1994). CGRP also modulates innate immunity by upregulating cyclic adenosine monophosphate and interleukin (IL)-8 expression, thereby promoting neutrophil chemotaxis to areas of acute inflammation in the cornea (Tran et al., 2000). Diminished CGRP levels have been correlated with dry eye severity (Lambiase et al., 2001), and as such, have been suggested by many researchers (Di Zazzo et al., 2019; Tamhane et al., 2019) as useful biomarkers of dry eye disease in tandem with SP. Moreover, CGRP serves vital functions in nociception of the central and peripheral nervous system (Schou et al., 2017), with the cornea being no exception. CGRP stimulates the release of nitric oxide from trigeminal ganglia in rat cornea (Vause and Durham, 2009) and algogenic factors such as bradykinin from satellite glial cells in mouse cornea (Ceruti et al., 2011). These effects help produce a favorable neurochemical environment that enhances neural activity. In addition, increased CGRP levels have been associated with increased corneal hyperalgesia following corneal injury in a rat model (Hegarty et al., 2018).
Another neuropeptide, vasoactive intestinal peptide (VIP), is expressed in limited quantities in the corneal nerves (Al-Aqaba et al., 2019), but nonetheless plays important roles in wound healing in the corneal epithelium. Zhang et al. (2020) demonstrated the role of VIP in modulating corneal epithelial healing and nerve regeneration, as well as exerting anti-inflammatory effects in a signaling pathway-dependent manner. Moreover, in their animal diabetic corneal model, exogenous VIP improved the epithelial healing, upregulated the wound-induced production of neurotrophic factors, and dampened the inflammatory response (Zhang et al., 2020). As an autocrine trophic factor in the corneal endothelium, VIP has also been shown to promote the survival of corneal endothelial cells under oxidative stress (Koh and Waschek, 2000), postulated to be via the upregulation of the anti-apoptotic factor Bcl-2 and differentiation marker N-cadherin in a kinase A inhibitor-dependent mechanism (Koh et al., 2009). Like VIP, neuropeptide Y (NPY) confers anti-inflammatory properties as well. It is the most abundant peptide in the central and peripheral nervous systems (Medeiros and Turner, 1996). It has been shown to serve bimodal functions, as both a strong negative regulator of T cells, and an activator of antigen-presenting cells in a mouse model (Wheway et al., 2005). The distribution of NPY in the human cornea was shown to be closely related to vascular distribution (Stone, 1986), and subsequently in a mouse model, its role as a stimulator of angiogenesis and angiogenesis-dependent wound healing was discovered (Ekstrand et al., 2003).
Neurotransmitters, in contrast to neuropeptides, are released rapidly at the synaptic junction, exerting short-term effects on a limited number of receptors. The catecholamines, norepinephrine and epinephrine, have been found to exert neurotrophic functions, aiding corneal wound healing through epithelial cell proliferation, migration and transcellular transport processes (Al-Aqaba et al., 2019). The neurotransmitter acetylcholine is present in high concentrations in the cornea, helping to maintain the ionic gradient during propagation of nerve impulses along an axon (Al-Aqaba et al., 2019). Acetylcholine stimulates corneal epithelial cell DNA synthesis, epithelial cell migration and keratocyte proliferation (Słoniecka et al., 2015). Acetylcholine also reduces apoptotic activity and corneal fibrosis by inhibiting the formation of myofibroblasts and dampening the excessive production of extracellular matrix (Słoniecka and Danielson, 2020). Corneal trigeminal axons also contain the enzyme acetylcholinesterase (AChE) (Al-Aqaba et al., 2019), which has been used as a technique for corneal nerve staining (Liu et al., 2021). This enzyme has been postulated to confer neurotrophic effects on the cornea, evidenced by the loss of corneal sensation when AChE production is repressed, and the absence of AChE and acetylcholine in denervated corneas (Al-Aqaba et al., 2019).
These neuromediators serve vital physiological roles in homeostatic processes of the cornea (Table 1). In corneas with normal physiological conditions, they help maintain essential cellular processes, including normal proliferation, apoptosis and neurotransmission. In corneas that have pathological changes or surgical insults including laser refractive surgery, they provide additional stimuli for healing and nerve regeneration, as well as the modulation of inflammatory processes (Gao et al., 2014; Zhang et al., 2016).
Table 1.
Summary of the physiological roles and translational applications of neuromediators in corneas
| Neuromediator | Functions | Translational applications | |
|---|---|---|---|
| Neurotrophin | Nerve growth factor | Epithelial cell migration, colony formation and proliferation in vivo
Corneal nociception in vitro Maintenance of corneal nerve density in vivo Induction of fibroblastic differentiation into myofibroblasts in vitro |
Nerve growth factor eye drops for neurotrophic keratopathy and post-refractive surgery regeneration (Nishida et al., 2007; Joo, 2014; Ma et al., 2014) Biomarker for dry eye disease (Tamhane et al., 2019) |
| Glial-derived neurotrophic factor | Epithelial cell migration, colony formation and proliferation in vitro | ||
| Brain-derived neurotrophic factor | Epithelial cell colony formation in vitro | ||
| Ciliary neurotrophic factor | Epithelial progenitor cells activation in vitro | ||
| Neurotrophin-3 | Corneal sensory and sympathetic nerve survival in vivo
Modulator of corneal nerve branching in vivo |
||
| Neuropeptide | Substance P | Epithelial cell migration, proliferation and adhesion in vivo
Mediator of reflex tear production in vivo Protection of corneal epithelial barrier in vitro Inhibition of epithelial cell apoptosis in vitro |
Substance P and insulin-like growth factor-1 eye drops for neurotrophic keratopathy (Bonini et al., 2018) Biomarker for dry eye disease and diabetic peripheral neuropathy (Chao et al., 2016; Tummanapalli et al., 2019) |
| Calcitonin gene-related peptide | Epithelial cell migration and differentiation in vivo
Innate immunity and neutrophil chemotaxis in vitro Vasoactive effects in vivo Corneal nociception in vitro |
Biomarker for dry eye disease (Di Zazzo et al., 2019; Tamhane et al., 2019) | |
| Vasoactive intestinal peptide | Corneal nerve regeneration in vivo
Upregulation of neurotrophin production in vivo Anti-inflammatory effects in vitro Corneal endothelial survival in vitro |
||
| Neuropeptide Y | Anti-inflammatory effects in vivo
Angiogenesis and angiogenesis-dependent wound healing in vivo |
||
| Neurotransmitter | Epinephrine and norepinephrine | Epithelial cell migration, proliferation and transcellular transport in vitro | |
| Acetylcholine | Epithelial cell DNA synthesis and migration Keratocyte proliferation in vitro Reduces corneal apoptosis and fibrosis in vitro Maintains ionic gradient during propagation of nerve impulse in vitro |
Corneal nerve staining to visualize corneal nerves distribution (Liu, 2020) |
Changes in Neuromediators Following Laser Refractive Surgery
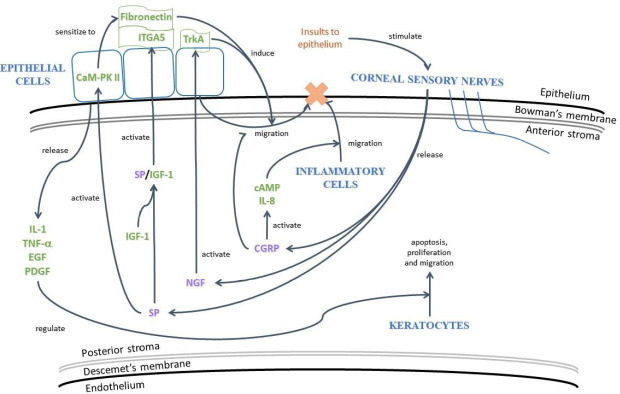
Corneal wound healing is associated with the postoperative refractive stability, predictability, visual outcomes and resultant patient satisfaction following refractive surgery (Liu et al., 2015). The healing process is complex, involving various interactions between cells, neuromediators, cytokines and chemokines on the cornea and ocular surface (Lim et al., 2016; Yawata et al. 2019). In response to stimuli such as FSL ablation or surgical incision, corneal sensory nerves of rats are stimulated to release the neuropeptides SP and CGRP, the principle mediators of neurogenic inflammation (Liu et al., 2020a). In rabbit cornea, SP interacts synergistically with IGF-1 to promote the migration of epithelial cells to the site of tissue injury (Al-Aqaba et al., 2019). Furthermore, through the activation of calmodulin-dependent protein kinase II in rabbit corneal epithelial cells, SP enhances cell migration via the induction of fibronectin and interleukin (IL)-6 (Yamada et al., 2005). Another neuropeptide, CGRP, modulates the innate immune response through the activation of cyclic adenosine monophosphate and IL-8, hence promoting the migration of inflammatory cells such as neutrophils to the wounded site. The epithelium also produces cytokines and growth factors, including IL-1, tumor necrosis factor-α, epidermal growth factor, and platelet-derived growth factor (Mohan et al., 2000). These inflammatory and trophic factors function to regulate apoptosis, proliferation and the migration of keratocytes after laser refractive surgery (Figure 2).
Figure 2.
The neurogenic inflammation process following injury to the cornea.
The neurogenic inflammation process following injury to the cornea. Damage of corneal nerves triggers inflammatory, neuroinflammatory and wound healing cascades in the cornea. cAMP: Cyclic adenosine monophosphate; CaM-PK II: calmodulin-dependent protein kinase II; CGRP: calcitonin gene-related peptide; EGF: epidermal growth factor; IGF-1: insulin-like growth factor-1; IL: interleukin; ITGA: integrin alpha; NGF: nerve growth factor; PDGF: platelet-derived growth factor; SP: substance P; TNF-α: tumor necrosis factor-α; TrkA: tropomyosin receptor kinase A.
Changes in various neuromediators have been observed following LASIK, photorefractive keratectomy, FLEx or SMILE. Mertaniemi et al. (1995) examined the effects of PRK on CGRP in a prospective study of 14 patients. Compared to the preoperative values, the release of CGRP in tears increased and peaked on postoperative day 2, and thereafter declined on day 7. It was postulated that the significant elevation of CGRP release on days 1–2 may be attributed to the secretion from damaged stromal nerves. Notably, despite the hypersecretion of tears postoperatively, there was no significant decrease in tear CGRP concentrations, signifying a simultaneous increased production of CGRP in tears possibly by the corneal sensory nerves. Similarly, in a longitudinal study comparing PRK and microkeratome LASIK, Lee et al. (2005) demonstrated that tear NGF/total tear protein (NGF/tP) ratio had the greatest increase in the immediate postoperative period following PRK, peaking at day 1, before decreasing from week 1 to month 6. LASIK resulted in a lower NGF/tP ratio as compared to the PRK procedure up to 1 month postoperatively, but there were no significant differences observed between both groups thereafter. At 6 months, the NGF/tP ratio returned to preoperative levels for both PRK and LASIK. In a similar study, Pérez-Santonja et al. (1999) showed that LASIK-treated eyes had significantly lower tear NGF levels compared to PRK during the first 3 months, and these differences became insignificant only after 6 months.
It has been hypothesized that the early postoperative differences in the tear NGF levels between PRK and LASIK are attributed to differences in the corneal wound healing processes. In PRK, the epithelium is removed, and the anterior stroma is ablated. On the other hand, LASIK relatively spares the epithelium, superficial stroma and Bowman’s layer, and only the anterior stroma is ablated fully. As a result, PRK stimulates a more enhanced inflammatory response, with the presence of cytokines such as IL-1β acting to upregulate NGF and tropomyosin receptor kinase A production (You et al., 2000). Similarly, the relative lack of an NGF surge in the immediate postoperative period in LASIK, as compared to epi-LASIK, which has greater damage to the corneal epithelium, has been reported in a rabbit model. This low NGF level might partially explain the slower corneal sensory recovery in LASIK than epi-LASIK (Wu et al., 2009). In a clinical study by Erie et al. (2005) on the evaluation of corneal nerve plexus, the subbasal nerve density did not recover to preoperative levels until 5 years after LASIK as compared with 2 years after PRK. As a consequence, exogenous NGF has been proposed as a potential treatment to improve corneal neural healing post-LASIK (Joo, 2004; Ma et al., 2014). Besides the neurotrophic effects reported in clinical trials of patients with NK, topical NGF has been demonstrated to enhance the regeneration of the subbasal nerve plexus, as well as corneal sensitivity after LASIK in rabbits (Joo, 2004; Ma et al., 2014). Moreover, NGF is involved in nociception in the cornea, with topical NGF shown to stimulate thermal and mechanical hyperalgesia in rats (Lewin et al., 1993). As a result, the lower NGF secretion after LASIK might therefore account for a diminished corneal sensitivity, compared to after PRK.
Several studies have also demonstrated increased tear IL-6 and SP levels in the early post-LASIK period of up to 3 months (Gao et al., 2014; Chao et al., 2015), and Gao et al., (2014) found the SP tear concentrations inversely associated with CNFD. Studies on the changes of CGRP level following LASIK have shown inconsistent results; Chao et al. (2015) reported that there were no significant elevations of CGRP in the postoperative period of up to 3 months, while another cross-sectional study by Chao et al., (2016) showed that the tear CGRP concentrations at postoperative 12 months were significantly higher than those of normal subjects.
Following SMILE, there are different tear neuromediator profiles, as well as ocular surface changes compared to LASIK. NGF and IL-6 concentrations in tears are increased in both SMILE and FSL-LASIK, and the levels return to baseline more rapidly in SMILE at 3 months (Gao et al., 2014). Several reasons underlie the differences in the neuromediator profiles in these two procedures. In LASIK, the stromal nerve fibers that run across the circumferential flap cut are truncated and resected, and the excimer laser ablation on the stromal bed further vaporizes deeper stromal nerves. On the contrary, in SMILE, only the nerves near the small incision and inside the refractive lenticule are interrupted, but the nerve bundles located outside the cap/lenticule area remained untouched (Mastropasqua, 2015). Our group has previously demonstrated that at 4 years postoperatively, SMILE patients had significant higher corneal nerve fiber length and fiber density as well as total corneal nerve branch density compared to LASIK patients, indicating better preservation and faster recovery of corneal nerves following SMILE (Liu et al., 2020a). Furthermore, LASIK has been proven to invoke a greater inflammatory response, extracellular matrix deposition and stromal interface reaction as compared to SMILE, hence resulting in a greater trigger of NGF release through neurogenic inflammation (Liu et al., 2016a).
As for FLEx and SMILE, the tear NGF levels are significantly higher at 1 day, 1 week and 1 month following FLEx, in comparison to SMILE. Transforming growth factor-β1 (TGF-β1) levels are also significantly higher in FLEx compared to SMILE, at 1 day and 1 week postoperatively. Zhang et al. (2016) postulated that the flap creation following FLEx stimulates a more extensive inflammatory reaction, and therefore a greater resultant secretion of NGF and cytokines. FLEx involves a 330° flap creation, while SMILE requires just a 30° incision. Furthermore, the authors also showed that the tear NGF, TGF-β1 and IL-1α levels were moderately and significantly correlated with the ocular surface disease index scores, corneal fluorescein staining and non-invasive TBUT values in both FLEx and SMILE groups. Table 2 summarizes the changes of tear neuromediators following laser refractive surgery.
Table 2.
Literature reporting the changes in tear neuromediators following refractive surgery
| Author | Study design | Surgery type and patient number | Findings on neuromediators |
|---|---|---|---|
| Mertaniemi et al. (1995) | Prospective | PRK (n = 14 eyes) | Tear CGRP increased at d 1, peaked at d 2, and decreased at d 7 after PRK. |
| Lee et al. (2005) | Prospective | PRK (n = 70 eyes) and microkeratome LASIK (n = 76 eyes) | Up to 1 mon postoperatively, LASIK resulted in significantly lower tear NGF/total protein ratio compared to PRK, but differences were not significant at 3 and 6 mon. |
| The mean NGF/total protein values at 1 d and 1 wk postoperatively correlated with corneal sensation, TBUT and Schirmer score at 6 months postoperatively for both groups. | |||
| Wu et al. (2009) | Rabbit model | Microkeratome LASIK (n = 24 eyes) and epi-LASIK (n = 24 eyes) | Tear NGF level was significantly lower in LASIK as compared to epi-LASIK on 1 d and 3 d, but differences were not significant at 7 d and 1 mon. |
| Chen et al. (2014) | Monkey model | Microkeratome LASIK (n=20 eyes) | NGF mRNA increased to 5.4-fold at day 3 and 2-fold above preoperative levels at d 7, and then returned to preoperative levels at 3 mon. |
| Increased NGF mRNA level correlated with CNFD at 3 and 7 d postoperatively. | |||
| Pérez-Santonja et al. (1999) | Prospective | PRK (n = 18 eyes) and microkeratome LASIK (n = 17 eyes) | Tear NGF level was significantly lower in LASIK than PRK during the first 3 mon, with no significant differences after 6 mon. |
| Chao et al. (2015) | Prospective | Microkeratome LASIK (n = 40 eyes) | Tear SP level increased during the early post-LASIK period, and did not return to the preoperative ranges until after 3 mon. |
| CNFD following LASIK was inversely correlated with dry eye symptoms and tear SP. | |||
| Chao et al. (2016) | Cross-sectional | Microkeratome LASIK (n = 40 eyes) | Tear SP level was positively and significantly associated with decreased CNFD and increased dry eye symptoms. |
| Tear CGRP level was significantly higher than that of controls at 12 mon. | |||
| Gao et al. (2014) | Prospective | FSL-LASIK (n = 64 eyes) and SMILE (n = 30 eyes) | Tear NGF peaked on the first day, recovered to preoperative values at 3 mon after SMILE, but remained slightly elevated after 3 mon following FSL-LASIK. |
| Zhang et al. (2016) | Prospective | FLEx (n = 18 eyes) and SMILE (n = 23 eyes) | FLEx resulted in significantly higher tear NGF level for the first 1 mon, and significantly higher TGF-β1 for the first 1 wk postoperatively, compared to SMILE. |
| NGF was significantly correlated with OSDI in both the FLEx and SMILE groups. NGF also had a significant association with NI-BUT in the FLEx and SMILE groups. |
CGRP: Calcitonin gene-related peptide; CNFD: corneal nerve fiber density; epi-LASIK: epi-laser in situ keratomileusis; FLEx: Femtosecond Lenticule Extraction; FSL-LASIK: Femtosecond Laser in Situ Keratomileusis; LASIK: Laser in Situ Keratomileusis; NGF: nerve growth factor; NI-BUT: non-invasive TBUT; OSDI: ocular surface disease index; PRK: photorefractive keratectomy; SMILE: Small Incision Lenticule Extraction; SP: substance P; TGF: transforming growth factor.
Correlation of Neuromediators with Clinical Dry Eye Parameters
The consequences of corneal denervation and neuroinflammation following refractive surgery are seen clinically on the ocular surface (Liu et al., 2020b). Refractive surgery results in a decrease in tear production, tear film quality, and blinking reflex, which are involved in the pathogenesis of dry eye disease. Tear inflammatory cytokines and neuromediators have been shown to be associated with the clinical evaluation of dry eye such as ocular surface or corneal staining, Schirmer test, TBUT, tear osmolarity, corneal sensitivity and ocular surface disease index scores, not only in post-laser refractive surgery patients but also in other ocular surface conditions. Studies in contact lens wearers have found that tear NGF correlated significantly and moderately with clinical grading of dry eye severity, ocular surface fluorescein staining and conjunctival hyperemia. Results on the CGRP levels showed opposite changes compared to those of NGF, whereby the CGRP concentration was correlated inversely with the severity of clinical dry eye (Lambiase et al., 2011). The decreased tear CGRP levels resulted from decreased mucin production by goblet cells, which is itself a feature of dry eye disease (Mantelli and Argüeso, 2008). Another study by Golebiowski et al. (2017) on contact lens wearers showed that the tear CGRP level was correlated with CNFD.
Increased tear interferon-γ concentrations are also correlated with ocular surface staining, Schirmer test scores and tear hyperosmolarity (Jackson et al., 2016), whereas elevated IL-6 levels are related to TBUT and disease severity in patients with dry eye (Yoon et al., 2007).
Given the correlation established between these neuromediators as well as inflammatory mediators and clinical parameters, these mediators may serve as useful biomarkers for ocular surface and corneal nerves status following laser refractive surgery. Moreover, by targeting these neuromediators, it may open a new avenue to enhance the corneal wound healing and nerve regeneration processes, as well as to alleviate complications such as dry eye following laser refractive surgery.
Conclusion
Neuromediators represent the complex interplay between corneal nerves, epithelial, stromal and endothelial cells, whose mutual release of neurotrophins, neuropeptides and neurotransmitters are vital to corneal homeostasis. Alterations in neuromediator profiles following laser refractive surgery are closely related to the surgical techniques and the severity of the corneal tissue insult induced. The growing understanding of neuromediators, as well as advancements in corneal nerve assessment, have inspired the use of tear neuromediators as potential biomarkers for ocular surface health and corneal nerve status following laser refractive surgery. Future directions include the validation of neuromediators as potential biomarkers, and the exploration of neuromediators as adjuncts to aid corneal regeneration after laser refractive surgery, or other ocular surface diseases such as neurotrophic keratopathy and dry eye.
Footnotes
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:None.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
C-Editors: Zhao M, Qiu Y; T-Editor: Jia Y
References
- 1.Al-Aqaba M A, Dhillon VK, Mohammed I, Said DG, Dua HS. Corneal nerves in health and disease. Prog Retin Eye Res. 2019;73:100762. doi: 10.1016/j.preteyeres.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Aloe L, Rocco ML, Balzamino BO, Micera A. Nerve growth factor: a focus on neuroscience and therapy. Curr Neuropharmacol. 2015;13:294–303. doi: 10.2174/1570159X13666150403231920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bandeira F, Yusoff NZ, Yam GH, Mehta JS. Corneal re-innervation following refractive surgery treatments. Neural Regen Res. 2019;14:557–565. doi: 10.4103/1673-5374.247421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett J, Boulter M, Baquet Z, Jones K. Trigeminal axon branching is reduced in the corneas of neurotrophin-3 hypomorphic mice. Invest Ophthalmol Vis Sci. 2002;43:3224–3224. [Google Scholar]
- 5.Bonini S, Lambiase A, Rama P, Sinigaglia F, Allegretti M, Chao W, Mantelli F. Phase II randomized, double-masked, vehicle-controlled trial of recombinant human nerve growth factor for neurotrophic keratitis. Ophthalmology. 2018;125:1332–1343. doi: 10.1016/j.ophtha.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 6.Bronzetti E, Artico M, Kovacs I, Felici LM, Magliulo G, Vignone D, D’Ambrosio A, Forte F, Di Liddo R, Feher J. Expression of neurotransmitters and neurotrophins in neurogenic inflammation of the rat retina. Eur J Histochem. 2007;51:251–260. [PubMed] [Google Scholar]
- 7.Ceruti S, Villa G, Fumagalli M, Colombo L, Magni G, Zanardelli M, Fabbretti E, Verderio C, van den Maagdenberg AM, Nistri A, Abbracchio MP. Calcitonin gene-related peptide-mediated enhancement of purinergic neuron/glia communication by the algogenic factor bradykinin in mouse trigeminal ganglia from wild-type and R192Q Cav2.1 Knock-in mice: implications for basic mechanisms of migraine pain. J Neurosci. 2011;31:3638–3649. doi: 10.1523/JNEUROSCI.6440-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chao C, Golebiowski B, Zhao X, Chen S, Zhou S, Stapleton F. Long-term effects of LASIK on corneal innervation and tear neuropeptides and the associations with dry eye. J Refract Surg. 2016;32:518–524. doi: 10.3928/1081597X-20160603-01. [DOI] [PubMed] [Google Scholar]
- 9.Chao C, Stapleton F, Zhou X, Chen S, Zhou S, Golebiowski B. Structural and functional changes in corneal innervation after laser in situ keratomileusis and their relationship with dry eye. Graefes Arch Clin Exp Ophthalmol. 2015;253:2029–2039. doi: 10.1007/s00417-015-3120-1. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Wei RH, Tan DT, Beuerman RW, Li W, Zhao S. Nerve growth factor expression and nerve regeneration in monkey corneas after LASIK. J Refract Surg. 2014;30:134–139. doi: 10.3928/1081597X-20140120-10. [DOI] [PubMed] [Google Scholar]
- 11.Di Zazzo A, Micera A, De Piano M, Cortes M, Bonini S. Tears and ocular surface disorders: usefulness of biomarkers. J Cell Physiol. 2019;234:9982–9993. doi: 10.1002/jcp.27895. [DOI] [PubMed] [Google Scholar]
- 12.Ekstrand AJ, Cao R, Bjorndahl M, Nystrom S, Jonsson-Rylander AC, Hassani H, Hallberg B, Nordlander M, Cao Y. Deletion of neuropeptide Y (NPY) 2 receptor in mice results in blockage of NPY-induced angiogenesis and delayed wound healing. Proc Natl Acad Sci U S A. 2003;100:6033–6038. doi: 10.1073/pnas.1135965100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erie JC, McLaren JW, Hodge DO, Bourne WM. Recovery of corneal subbasal nerve density after PRK and LASIK. Am J Ophthalmol. 2005;140:1059–1064. doi: 10.1016/j.ajo.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 14.Fuest M, Liu YC, Coroneo MT, Mehta JS. Femtosecond laser assisted pterygium surgery. Cornea. 2017;36:889–892. doi: 10.1097/ICO.0000000000001230. [DOI] [PubMed] [Google Scholar]
- 15.Fuest M, Liu YC, Yam GH, Teo EP, Htoon HM, Coroneo MT, Mehta Femtosecond laser-assisted conjunctival autograft preparation for pterygium surgery. Ocul Surf. 2017;15:211–217. doi: 10.1016/j.jtos.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Gao S, Li S, Liu L, Wang Y, Ding H, Li L, Zhong X. Early changes in ocular surface and tear inflammatory mediators after small-incision lenticule extraction and femtosecond laser-assisted laser in situ keratomileusis. PLoS One. 2014;9:e107370. doi: 10.1371/journal.pone.0107370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Hirschfeld J, Lopez-Briones LG, Belmonte C. Neurotrophic influences on corneal epithelial cells. Exp Eye Res. 1994;59:597–605. doi: 10.1006/exer.1994.1145. [DOI] [PubMed] [Google Scholar]
- 18.Golebiowski B, Chao C, Stapleton F, Jalbert I. Corneal nerve morphology, sensitivity, and tear neuropeptides in contact lens wear. Optom Vis Sci. 2017;94:534–542. doi: 10.1097/OPX.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 19.Han SB, Liu YC, Mohamed-Noriega K, Mehta JS. Application of femtosecond laser in anterior segment surgery. J Ophthalmol. 2020;2020:8263408. doi: 10.1155/2020/8263408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He J, Bazan E. Neuroanatomy and neurochemistry of mouse cornea. Invest Ophthalmol Vis Sci. 2016;57:664–674. doi: 10.1167/iovs.15-18019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hegarty DM, Hermes SM, Morgan MM, Aicher SA. Acute hyperalgesia and delayed dry eye after corneal abrasion injury. Pain Rep. 2018;3:e.664–e.664. doi: 10.1097/PR9.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson DC, Zeng W, Wong CY, Mifsud EJ, Williamson NA, Ang CS, Vingrys AJ, Downie LE. Tear interferon-gamma as a biomarker for evaporative dry eye disease. Invest Ophthalmol Vis Sci. 2016;57:4824–4830. doi: 10.1167/iovs.16-19757. [DOI] [PubMed] [Google Scholar]
- 23.Joo MJ, Yuhan KR, Hyon JY, Lai H, Hose S, Sinha D, O’Brien TP. The effect of nerve growth factor on corneal sensitivity after laser in situ keratomileusis. Arch Ophthalmol. 2004;122:1338–1341. doi: 10.1001/archopht.122.9.1338. [DOI] [PubMed] [Google Scholar]
- 24.Kim TI, Alió Del Barrio JL, Wilkins M, Cochener B, Ang M. Refractive surgery. Lancet. 2019;393:2085–2098. doi: 10.1016/S0140-6736(18)33209-4. [DOI] [PubMed] [Google Scholar]
- 25.Koh SW, Cheng MJ, Dodson RM, Ku CYT, Abbondandolo CJ. VIP down-regulates the inflammatory potential and promotes survival of dying (neural crest-derived) corneal endothelial cells ex vivo: necrosis to apoptosis switch and up-regulation of Bcl-2 and N-cadherin. J Neurochem. 2009;109:792–806. doi: 10.1111/j.1471-4159.2009.06012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koh SW, Waschek JA. Corneal endothelial cell survival in organ cultures under acute oxidative stress: effect of VIP. Invest Ophthalmol Vis Sci. 2000;41:4085–4092. [PubMed] [Google Scholar]
- 27.Lambiase A, Bonini S, Micera A, Rama P, Bonini S, Aloe L. Expression of nerve growth factor receptors on the ocular surface in healthy subjects and during manifestation of inflammatory diseases. Invest Ophthalmol Vis Sci. 1998;39:1272–1275. [PubMed] [Google Scholar]
- 28.Lambiase A, Manni L, Bonini S, Rama P, Micera A, Aloe L. Nerve growth factor promotes corneal healing: structural, biochemical, and molecular analyses of rat and human corneas. Invest Ophthalmol Vis Sci. 2000;41:1063–1069. [PubMed] [Google Scholar]
- 29.Lambiase A, Micera A, Sacchetti M, Cortes M, Mantelli F, Bonini S. Alterations of tear neuromediators in dry eye disease. Arch Ophthalmol. 2011;129:981–986. doi: 10.1001/archophthalmol.2011.200. [DOI] [PubMed] [Google Scholar]
- 30.Lee HK, Lee KS, Kim HC, Lee SH, Kim EK. Nerve growth factor concentration and implications in photorefractive keratectomy vs laser in situ keratomileusis. Am J Ophthalmol. 2005;139:965–971. doi: 10.1016/j.ajo.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 31.Lewin GR, Ritter AM, Mendell LM. Nerve growth factor-induced hyperalgesia in the neonatal and adult rat. J Neurosci. 1993;13:2136–2148. doi: 10.1523/JNEUROSCI.13-05-02136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim RR, Tan A, Liu YC, Barathi VA, Mohan RR, Mehta JS, Chaurasia SS. ITF2357 transactivates Id3 and regulate TGFb/BMP7 signaling pathways to attenuate corneal fibrosis. Sci Rep. 2016;6:20841. doi: 10.1038/srep20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Q. Houston: University of Houston; 2010. The biophysiology and pathophysiology of nerve growth factor at the ocular surface. [Google Scholar]
- 34.Liu YC, Ang HP, Teo EP, Lwin NC, Yam GH, Mehta JS. Wound healing profiles of hyperopic-small incision lenticule extraction (SMILE) Sci Rep. 2016a;6:29802. doi: 10.1038/srep29802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu YC, Jung A, Jia AJS, Yang LWY, Mehta JS. Cross-sectional study on denervation in contralateral eyes following small incision lenticule extraction versus laser-assisted in-situ keratomileusis. J Refract Surg. 2020a;36:653–660. doi: 10.3928/1081597X-20200730-01. [DOI] [PubMed] [Google Scholar]
- 36.Liu YC, Lin MTY, Mehta JS. Analysis of corneal nerve plexus in corneal confocal microscopy images. Neural Regen Res. 2021;16:690–691. doi: 10.4103/1673-5374.289435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu YC, Rosman M, Mehta JS. Amsterdam, Netherlands: Elsevier; 2016b. Small Incision Lenticule Extraction (SMILE) [Google Scholar]
- 38.Liu YC, Tan DTH, Mehta JS. Wound healing after ReLEx Surgery. In: Sekundo W, editor. Small incision lenticule extraction: Principles, Techniques, Complication management and future concepts. Berlin Heidelberg New York: Springer; 2015. pp. 13–25. [Google Scholar]
- 39.Liu YC, Teo EPW, Ang HP, Seah XY, Lwin NC, Yam GHF, Mehta JS. Biological corneal inlay for presbyopia derived from small incision lenticule extraction (SMILE) Sci Rep. 2018;8:1831. doi: 10.1038/s41598-018-20267-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu YC, Wilkins M, Kim T, Malyugin B, Mehta JS. Cataracts. Lancet. 2017;390:600–612. doi: 10.1016/S0140-6736(17)30544-5. [DOI] [PubMed] [Google Scholar]
- 41.Liu YC, Wittwer VV, Yusoff NZM, Lwin CN, Seah XY, Mehta JS, Seiler T. Intraoperative optical coherence tomography-guided femtosecond laser-assisted deep anterior lamellar keratoplasty. Cornea. 2019;38:648–653. doi: 10.1097/ICO.0000000000001851. [DOI] [PubMed] [Google Scholar]
- 42.Liu YC, Yam HF, Lin TY, Teo E, Koh SW, Deng L, Zhou L, Tong L, Mehta JS. Comparison of tear proteomic and neuromediator profiles changes between small incision lenticule (SMILE) and femtosecond laser-assisted in-situ keratomileusis (LASIK) J Adv Res. 2020b. e-pub. https://doi.org/10.1016/j.jare.2020.11.001 . [DOI] [PMC free article] [PubMed]
- 43.Lou L, Yao C, Jin Y, Perez V, Ye J. Global patterns in health burden of uncorrected refractive error. Invest Ophthalmol Vis Sci. 2016;57:6271–6277. doi: 10.1167/iovs.16-20242. [DOI] [PubMed] [Google Scholar]
- 44.Ma K, Yan N, Huang Y, Cao G, Deng J, Deng Y. Effects of nerve growth factor on nerve regeneration after corneal nerve damage. Int J Clin Exp Med. 2014;7:4584–4589. [PMC free article] [PubMed] [Google Scholar]
- 45.Mansoor H, Tan HC, Lin MT, Mehta JS, Liu YC. Diabetic corneal neuropathy. J Clin Med. 2020;9:3956. doi: 10.3390/jcm9123956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mantelli F, Argüeso P. Functions of ocular surface mucins in health and disease. Curr Opin Allergy Clin Immunol. 2008;8:477–483. doi: 10.1097/ACI.0b013e32830e6b04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marfurt CF, Murphy CJ, Florczak JL. Morphology and neurochemistry of canine corneal innervation. Invest Ophthalmol Vis Sci. 2001;42:2242–2251. [PubMed] [Google Scholar]
- 48.Mastropasqua L, Mario N. Corneal nerve and keratocyte response to ReLEx surgery. In: Sekundo W, editor. Small Incision Lenticule Extraction (SMILE): Principles, Techniques, Complication management and Future concepts. New York: Springer; 2015. pp. 27–43. [Google Scholar]
- 49.Medeiros Mdos S, Turner AJ. Metabolism and functions of neuropeptide Y. Neurochem Res. 1996;21:1125–1132. doi: 10.1007/BF02532423. [DOI] [PubMed] [Google Scholar]
- 50.Mertaniemi P, Ylätupa S, Partanen P, Tervo T. Increased release of immunoreactive calcitonin gene-related peptide (CGRP) in tears after excimer laser keratectomy. Exp Eye Res. 1995;60:659–665. doi: 10.1016/s0014-4835(05)80007-7. [DOI] [PubMed] [Google Scholar]
- 51.Mohan RR, Mohan RR, Kim WJ, Wilson SE. Modulation of TNF-alpha-induced apoptosis in corneal fibroblasts by transcription factor NF-kappaB. Invest Ophthalmol Vis Sci. 2000;41:1327–1336. [PubMed] [Google Scholar]
- 52.Müller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 53.Murphy CJ, Campbell S, Araki-Sasaki K, Marfurt CF. Effect of norepinephrine on proliferation, migration, and adhesion of SV-40 transformed human corneal epithelial cells. Cornea. 1998;17:529–536. doi: 10.1097/00003226-199809000-00011. [DOI] [PubMed] [Google Scholar]
- 54.Nakamura M, Chikama T, Nishida T. Up-regulation of integrin alpha 5 expression by combination of substance P and insulin-like growth factor-1 in rabbit corneal epithelial cells. Biochem Biophys Res Commun. 1998;246:777–782. doi: 10.1006/bbrc.1998.8704. [DOI] [PubMed] [Google Scholar]
- 55.Nishida T, Chikama T, Morishige N, Yanai R, Yamada N, Saito J. Persistent epithelial defects due to neurotrophic keratopathy treated with a substance p-derived peptide and insulin-like growth factor 1. Jpn J Ophthalmol. 2007;51:442–447. doi: 10.1007/s10384-007-0480-z. [DOI] [PubMed] [Google Scholar]
- 56.Nishida T, Nakamura M, Ofuji K, Reid TW, Mannis MJ, Murphy CJ. Synergistic effects of substance P with insulin-like growth factor-1 on epithelial migration of the cornea. J Cell Physiol. 1996;169:159–166. doi: 10.1002/(SICI)1097-4652(199610)169:1<159::AID-JCP16>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 57.Pérez-Santonja JJ, Sakla HF, Cardona C, Chipont E, Alió JL. Corneal sensitivity after photorefractive keratectomy and laser in situ keratomileusis for low myopia. Am J Ophthalmol. 1999;127:497–504. doi: 10.1016/s0002-9394(98)00444-9. [DOI] [PubMed] [Google Scholar]
- 58.Salomão MQ, Wilson SE. Femtosecond laser in laser in situ keratomileusis. J Cataract Refract Surg. 2010;36:1024–1032. doi: 10.1016/j.jcrs.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sandoval HP, Donnenfeld ED, Kohnen T, Lindstrom RL, Potvin R, Tremblay DM, Solomon KD. Modern laser in situ keratomileusis outcomes. J Cataract Refract Surg. 2016;42:1224–1234. doi: 10.1016/j.jcrs.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 60.Schou WS, Ashina S, Amin FM, Goadsby PJ, Ashina M. Calcitonin gene-related peptide and pain: a systematic review. J Headache Pain. 2017;18:34. doi: 10.1186/s10194-017-0741-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sekundo W, Kunert K, Russmann C, Gille A, Bissmann W, Stobrawa G, Sticker M, Bischoff M, Blum M. First efficacy and safety study of femtosecond lenticule extraction for the correction of myopia: six-month results. J Cataract Refract Surg. 2008;34:1513–1520. doi: 10.1016/j.jcrs.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 62.Słoniecka M, Backman LJ, Danielson P. Acetylcholine enhances keratocyte proliferation through muscarinic receptor activation. Int Immunopharmacol. 2015;29:57–62. doi: 10.1016/j.intimp.2015.05.039. [DOI] [PubMed] [Google Scholar]
- 63.Słoniecka M, Danielson P. Acetylcholine decreases formation of myofibroblasts and excessive extracellular matrix production in an in vitro human corneal fibrosis model. J Cell Mol Med. 2020;24:4850–4862. doi: 10.1111/jcmm.15168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stone RA. Neuropeptide Y and the innervation of the human eye. Exp Eye Res. 1986;42:349–355. doi: 10.1016/0014-4835(86)90028-x. [DOI] [PubMed] [Google Scholar]
- 65.Tamhane M, Cabrera-Ghayouri S, Abelian G, Viswanath V. Review of biomarkers in ocular matrices: challenges and opportunities. Pharm Res. 2019;36:40. doi: 10.1007/s11095-019-2569-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tran MT, Ritchie MH, Lausch RN, Oakes JE. Calcitonin gene-related peptide induces IL-8 synthesis in human corneal epithelial cells. J Immunol. 2000;164:4307–4312. doi: 10.4049/jimmunol.164.8.4307. [DOI] [PubMed] [Google Scholar]
- 67.Tummanapalli SS, Willcox MDP, Issar T, Yan A, Pisarcikova J, Kwai N, Poynten AM, Krishnan AV, Markoulli M. Tear film substance P: A potential biomarker for diabetic peripheral neuropathy. Ocul Surf. 2019;17:690–698. doi: 10.1016/j.jtos.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 68.Uusitalo H, Krootila K, Palkama A. Calcitonin gene-related peptide (CGRP) immunoreactive sensory nerves in the human and guinea pig uvea and cornea. Exp Eye Res. 1989;48:467–475. doi: 10.1016/0014-4835(89)90030-4. [DOI] [PubMed] [Google Scholar]
- 69.Vause CV, Durham PL. CGRP stimulation of iNOS and NO release from trigeminal ganglion glial cells involves mitogen-activated protein kinase pathways. J Neurochem. 2009;110:811–821. doi: 10.1111/j.1471-4159.2009.06154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wheway J, Mackay CR, Newton RA, Sainsbury A, Boey D, Herzog H, Mackay F. A fundamental bimodal role for neuropeptide Y1 receptor in the immune system. J Exp Med. 2005;202:1527–1538. doi: 10.1084/jem.20051971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilkinson GA, Fariñas I, Backus C, Yoshida CK, Reichardt LF. Neurotrophin-3 is a survival factor in vivo for early mouse trigeminal neurons. J Neurosci. 1996;16:7661–7669. doi: 10.1523/JNEUROSCI.16-23-07661.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu Y, Chu R, Zhou X, Dai J, Qu X. Determination of the nerve growth factor level in the central cornea after LASIK and Epi-LASIK treatment in a rabbit model system. Cornea. 2009;28:1144–1148. doi: 10.1097/ICO.0b013e3181a2a7e3. [DOI] [PubMed] [Google Scholar]
- 73.Yamada M, Ogata M, Kawai M, Mashima Y, Nishida T. Substance P in human tears. Cornea. 2003;22:S48–54. doi: 10.1097/00003226-200310001-00007. [DOI] [PubMed] [Google Scholar]
- 74.Yamada N, Yanai R, Inui M, Nishida T. Sensitizing effect of substance P on corneal epithelial migration induced by IGF-1, fibronectin, or interleukin-6. Invest Ophthalmol Vis Sci. 2005;46:833–839. doi: 10.1167/iovs.04-0775. [DOI] [PubMed] [Google Scholar]
- 75.Yawata N, Awate S, Liu YC, Yuan S, Woon K, Siak J, Kawana YI, Sonoda KH, Mehta J, Yawata M. Kinetics of tear fluid proteins after endothelial keratoplasty and predictive factors for recovery from corneal haze. J Clin Med. 2019;9:63. doi: 10.3390/jcm9010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoon KC, Jeong IY, Park YG, Yang SY. Interleukin-6 and tumor necrosis factor-alpha levels in tears of patients with dry eye syndrome. Cornea. 2007;26:431–437. doi: 10.1097/ICO.0b013e31803dcda2. [DOI] [PubMed] [Google Scholar]
- 77.You L, Ebner S, Kruse FE. Glial cell–derived neurotrophic factor (GDNF)–induced migration and signal transduction in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2001;42:2496–2504. [PubMed] [Google Scholar]
- 78.You L, Kruse FE, Völcker HE. Neurotrophic factors in the human cornea. Invest Ophthalmol Vis Sci. 2000;41:692–702. [PubMed] [Google Scholar]
- 79.Zhang C, Ding H, He M, Liu L, Liu L, Li G, Niu B, Zhong X. Comparison of early changes in ocular surface and inflammatory mediators between femtosecond lenticule extraction and small-incision lenticule extraction. PLoS One. 2016;11:e0149503. doi: 10.1371/journal.pone.0149503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Y, Gao N, Wu L, Lee PSY, Me R, Dai C, Xie L, Yu FX. Role of VIP and sonic hedgehog signaling pathways in mediating epithelial wound healing, sensory nerve regeneration, and their defects in diabetic corneas. Diabetes. 2020;69:1549. doi: 10.2337/db19-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou Q, Chen P, Di G, Zhang Y, Wang Y, Qi X, Duan H, Xie L. Ciliary neurotrophic factor promotes the activation of corneal epithelial stem/progenitor cells and accelerates corneal epithelial wound healing. Stem Cells. 2015;33:1566–1576. doi: 10.1002/stem.1942. [DOI] [PubMed] [Google Scholar]