Abstract
Cutaneous pseudolymphomas are a group of benign lymphocyte-rich infiltrates that can mimic cutaneous lymphomas either clinically and/or histologically. Idiopathic T-cell pseudolymphoma (TCPL) usually presents as a solitary nodule or plaque on the trunk or head. A clinicopathologic correlation is mandatory to arrive at a final diagnosis and rule out true lymphomas. There are only sparse dermoscopic reports on cutaneous pseudolymphomas. Hereby, we describe the clinical and dermoscopic features of a case of idiopathic TCPL in a 26-year-old man who presented with an asymptomatic thin reddish-brown “table tennis racquet”-shaped plaque on the right inframammary area.
Keywords: Cutaneous, dermoscopy, pseudolymphoma, T-lymphocytes
Introduction
Cutaneous pseudolymphomas are a group of benign lymphocyte-rich infiltrates, which mimic cutaneous lymphomas either clinically and/or histopathologically. Clinicopathologic correlation is mandatory to arrive at the final diagnosis and rule out true lymphomas.[1] The diagnosis of idiopathic cutaneous T-cell pseudolymphoma (TCPL), in contrast to other variants of TCPL, is challenging due to the lack of any characteristic clinical morphology and underlying association.[2,3] Hereby, we describe the clinical and dermoscopic features of a case of idiopathic TCPL that was successfully excised without any recurrence.
Case Report
A 26-year-old man with skin phototype IV presented with two months history of a slowly growing asymptomatic lesion on the right side of the chest. He denied any history of prior insect bite, trauma, drug intake, or topical application at the site. It was not associated with any systemic features like fever, night sweats, anorexia, or weight loss. Cutaneous examination revealed a solitary firm, reddish-brown flat-topped thin plaque of size 1.5 cm × 1 cm on the right inframammary area. The “table tennis racquet”-shaped plaque had an ill-defined margin, and the surface had a cobblestone appearance [Figure 1]. Other mucocutaneous, general, and systemic examinations were within normal limits. Differential diagnoses of superficial basal cell carcinoma, cutaneous lymphoma, and pseudolymphoma were considered. Dermoscopic examination (HEINE DELTA20®, 10× magnification) under nonpolarized mode revealed a cobblestone pattern, which was comprised of multiple round to oval salmon-colored to yellowish-orange structureless areas. The structureless areas were separated from each other by thick gray-white lines arranged in a network-like pattern. The vascular structures, linear, linear branching, and curved vessels were noticed to cross over the surface of the round to oval structureless areas. Other features noticed were fine brown peppering, vascular blotches, normal eccrine duct opening as white globules, and vellus hair follicle with a perifollicular white rim [Figure 2]. Laboratory investigations were within normal limits. Histology of the excised plaque showed multinodular dome-shaped dense papillary dermal and upper perivascular lymphohistiocytic infiltration with occasional eosinophils. The nodular infiltrate was expanding the papillary dermis and had a well-demarcated lateral and lower border. The lymphocytes were small to medium-sized without any significant pleomorphism, mitosis, or necrosis. The overlying epidermis displayed irregular acanthosis and elongated rete ridges that bordered the expanded papillary dermis [Figure 3a and b]. On immunohistochemistry, the lymphocytes were immunoreactive predominantly for CD3 and few cells for CD20 [Figure 3c and d]. Immunohistochemistry for the immunoglobulin light chain was positive for both kappa and lambda. The diagnosis of cutaneous TCPL, idiopathic subtype, was made.
Figure 1.
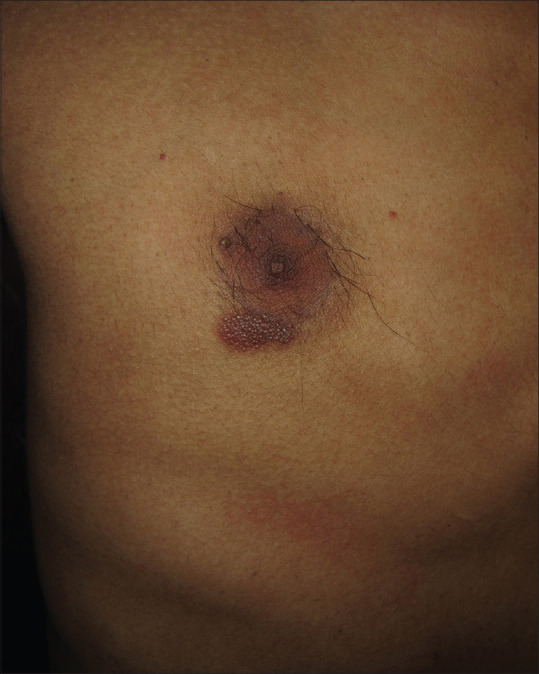
Solitary “table tennis racquet”-shaped thin reddish-brown plaque on the right inframammary area with a cobblestone appearance of the surface
Figure 2.

Dermoscopic examination (HEINE DELTA20® Dermatoscope, 10× magnification) under nonpolarized contact dermoscopy demonstrates multiple round to oval salmon-colored to yellowish-orange structureless areas in a cobblestone pattern separated by thick gray-white lines arranged in a network-like pattern (blue arrow), along with fine brown peeping, vascular blotch (asterisk), linear (black arrow), linear branching (red arrow), and linear curved vessels
Figure 3.

(a) Histology showing dense multinodular dome-shaped lymphohistiocytic infiltration in the papillary dermis. The elongated rete ridges bordering the expanded papillary dermis (H and E, ×50). (b) Bland lymphocytes with occasional eosinophils (H and E, ×400). (c) Lymphocytes with dominant positive immunoreactivity for CD3 (IHC, ×100). (d) Lymphocytes with occasional positive immunoreactivity for CD20 (IHC, ×100)
Discussion
Cutaneous pseudolymphomas are a heterogeneous group of benign lymphoproliferative disorders of either B-or-T-cell origin. Cutaneous TCPL is divided into the following subtypes based upon the underlying causes: (i) idiopathic, in which there is no associated identifiable cause; (ii) lymphomatoid contact dermatitis, due to contact allergen like nickel, cobalt, gold, rubber chemicals, dyes, and preservatives; (iii) lymphomatoid drug eruption, due to drugs like anticonvulsant, antidepressant, antihypertensive, beta-blockers, calcium channel blockers, diuretics, and antibiotics; (iv) actinic reticuloid, a severe form of photosensitivity reaction to UVB, UVA, and sometimes to the visible light; (v) persistent arthropod bite reaction; and (vi) nodular scabies.[3,4]
The idiopathic subtype of TCPL commonly presents as asymptomatic solitary erythematous to violaceous nodule or plaques on the head or trunk. The diagnosis can be challenging as it can mimic both cutaneous lymphomas and nonlymphomatous lesions like amelanotic melanoma, basal cell carcinoma, squamous cell carcinoma, and adnexal tumors.[3,4] The index case had a “table tennis racquet”-shaped thin reddish-brown plaque with cobblestoning, located on the inframammary area.
Dermoscopic features of TCPL are sparsely reported.[5,6] In the index case, multiple round to oval salmon-colored to yellowish-orange structureless areas that together imparted a cobblestone pattern correspond to the nodular lymphoid aggregate in the papillary dermis, and the thick gray-white lines to the pigmented and elongated rete ridges that bordered the lymphoid aggregates. Similarly, Geller et al. reported a constant orange/salmon color along with follicular plugs in 14 cases of lymphoproliferative disorders that histopathologically correlated to dense dermal lymphocytic infiltration and follicular plug, respectively.[5] The normal eccrine and follicular opening in the index case suggest that these structures are unaffected and not obscured by the pseudolymphomatous infiltration. A pink-orange color has been described in a case of pseudolymphoma with mixed infiltrate of T and B lymphocytes.[6] A case of B-cell pseudolymphoma revealed a pinkish background with white reticular lines and fine linear vessels.[7] Similarly, pseudolymphomatous folliculitis showed perifollicular and follicular yellowish spots, follicular red dots, and prominent arborizing vessels.[8] Dermoscopic examination may not help distinguish between lymphoma and pseudolymphomas as both have dense lymphocytic infiltration, which correlates to the salmon-color to yellow-orange structureless area.[6]
A histopathological examination, along with immunohistochemistry, is always mandatory for the diagnosis of cutaneous pseudolymphomas. Idiopathic TCPL can display either; a superficial band-like pattern, characterized by a dense superficial lymphocytic infiltration with sharply demarcated lateral and lower borders, or; a nodular/diffuse pattern, typified by dense, dermal diffuse or nodular infiltrates that extend into the subcutis without any epidermotropism. The infiltrate comprises of predominant small to medium-sized CD3+, CD4+, CD8–, and CD30- pleomorphic T cells with scattered medium to large-sized atypical T-cells which typically express programmed death-1 (PD-1), Bcl-6, and CXCL13, suggesting a common follicular helper T-cell phenotype.[2,4] The main challenge is to exclude cutaneous T-cell lymphomas (CTCL), which is marked by the presence of medium to large atypical lymphocytes with high proliferative index, epidermotropism, Pautrier's microabscess, and lymphocyte tagging. The presence of uniform round small to medium-sized lymphocytes without pleomorphism, absence of lymphocytes with cerebriform nucleus, a mixed inflammatory infiltrate consisting of both T-and-B-cells, eosinophils, plasma cells, and histiocytes, including occasional multinucleate giant cells, and low proliferative index favor the diagnosis of TCPL.[4] A superficial and deep perivascular lymphocytic infiltration in polymorphous light eruption can mimic pseudolymphoma; however, the presence of spongiosis, upper dermal edema, and lymphocytic exocytosis support the diagnosis of the former.[9]
At times, it is impossible to distinguish the benign TCPL from low-grade lymphoma pathologically, and additional help of immunohistochemistry and molecular method is required. The loss of CD7 (occurs early) and pan-T-cell markers, CD2, and CD5 (occurs later) and a positive T-cell receptor gene rearrangement supports the diagnosis of T-cell lymphoma. However, clonality analysis results should be interpreted based upon the clinical and pathological picture, as benign lymphoproliferative lesions can have foci of monoclonal cells, and neoplastic lymphoma may not demonstrate clonality.[2] Although rare, a small subset of cutaneous pseudolymphoma can progress to lymphoma. The cytological changes over time that point to the transformation are progressive atypia, increased cellular size, variation in shape, and nuclear qualities. However, it is still debatable whether the frank lymphoma is a progression from the benign pseudolymphoma or was an earlier missed lymphoma.[10]
The self-resolution of idiopathic TCPL following the skin biopsy has been reported. When the lesion is persistent, it can be treated with topical or intralesional steroids, surgical excision, and rarely radiotherapy. The other therapeutic modalities are topical tacrolimus, imiquimod, UVA1, Psoralen and UVA, 5-Aminolaevulinic acid-photodynamic therapy, and oral hydroxychloroquine.[11,12]
In conclusion, we are reporting the clinical and dermoscopic features of a case of idiopathic TCPL that was successfully excised without any recurrence. Dermoscopic features, a salmon-colored/yellowish-orange structureless area along with overlying linear vessels, reflect the underlying pathology of TCPL and can provide additional information to the diagnosis of TCPL in an appropriate clinical setting.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initial s will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Mitteldorf C, Kempf W. Cutaneous pseudolymphoma. Surg Pathol Clin. 2017;10:455–76. doi: 10.1016/j.path.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Mitteldorf C, Kempf W. Cutaneous pseudolymphoma-A review on the spectrum and a proposal for a new classification. J Cutan Pathol. 2020;47:76–97. doi: 10.1111/cup.13532. [DOI] [PubMed] [Google Scholar]
- 3.Iwai Y, Ibusuki A, Kawai K. Idiopathic cutaneous T-cell pseudolymphoma with prominent granulomatous reaction. J Cutan Immunol Allergy. 2020;3:19–20. [Google Scholar]
- 4.Willemze R. Post TW, editor. Cutaneous T cell pseudolymphomas. UpToDate. [Last accessed on 2020 October 24]. Available from: https://www.uptodate.com/contents/cutaneous-t-cell-pseudolymphomas?search=Cutaneous%20T%20cell%20pseudolymphomas&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 .
- 5.Geller S, Navarrete-Dechent C, Myskowski PL. Dermoscopy in lymphoproliferative disorders-experience from a cutaneous lymphoma clinic in a tertiary cancer center. J Am Acad Dermatol. 2019;80:e171–2. doi: 10.1016/j.jaad.2018.12.064. [DOI] [PubMed] [Google Scholar]
- 6.Bombonato C, Pampena R, Lallas A, Giovanni P, Longo C. Dermoscopy of lymphomas and pseudolymphomas. Dermatol Clin. 2018;36:377–88. doi: 10.1016/j.det.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Namiki T, Miura K, Tokoro S, Tanaka M, Yokozeki H. Dermoscopic features of lymphocytoma cutis: A case report of a representative dermoscopic feature. J Dermatol. 2016;43:1367–8. doi: 10.1111/1346-8138.13382. [DOI] [PubMed] [Google Scholar]
- 8.Fujimura T, Hidaka T, Hashimoto A, Aiba S. Dermoscopy findings of pseudolymphomatous folliculitis. Case Rep Dermatol. 2012;4:154–7. doi: 10.1159/000341194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kontos AP, Cusack CA, Chaffins M, Lim HW. Polymorphous light eruption in African Americans: Pinpoint papular variant. Photodermatol Photoimmunol Photomed. 2002;18:303–6. doi: 10.1034/j.1600-0781.2002.02779.x. [DOI] [PubMed] [Google Scholar]
- 10.Kulow BF, Cualing H, Steele P, Van Horn J, Breneman JC, Mutasim DF, et al. Progression of cutaneous B-cell pseudolymphoma to cutaneous B-cell lymphoma. J Cutan Med Surg. 2002;6:519–28. doi: 10.1007/s10227-001-0133-7. [DOI] [PubMed] [Google Scholar]
- 11.Miguel D, Peckruhn M, Elsner P. Treatment of cutaneous pseudolymphoma: A systematic review. Acta Derm Venereol. 2018;98:310–7. doi: 10.2340/00015555-2841. [DOI] [PubMed] [Google Scholar]
- 12.Koguchi H, Arita K, Nakazato S, Moriuchi R, Yamane N, Shinkuma S, et al. An erythematous plaque on the breast: A quiz. Solitary T-cell pseudolymphoma, superficial type. Acta Derm Venereol. 2013;93:763, 608. doi: 10.2340/00015555-1527. [DOI] [PubMed] [Google Scholar]


