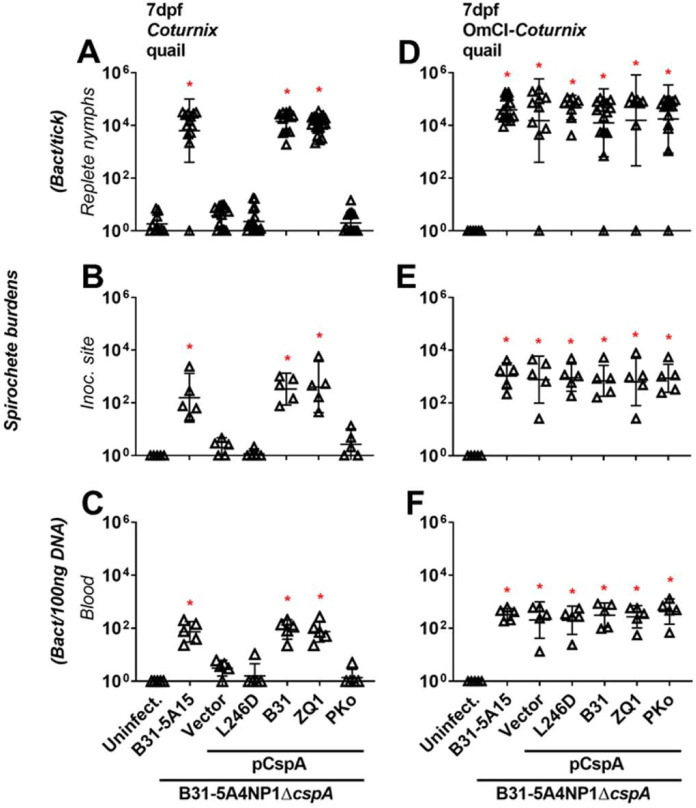
Fig 3. Polymorphic quail FH-binding activity of CspA confers the distinct transmissibility of Lyme borreliae to quail.
Ixodes scapularis nymphs infected with WT B. burgdorferi B31-5A15 (“B31-5A15”), B. burgdorferi B31-5A4NP1ΔcspA transformed with an empty shuttle vector (“Vector”), or this deletion strain producing a mutated variant of CspA from B. burgdorferi B31 selectively devoid of quail FH binding activity (“L246D”), WT B. burgdorferi B31 (“B31”), B. garinii ZQ1 (“ZQ1”), or B. afzelii PKo (“PKo”) were allowed to feed on (A-C) untreated or (D-F) OmCI-treated quail. Uninfected nymphs and quail tissues were included as control (“Uninfect.”). Fed nymphs were collected upon repletion, and blood and tissues were collected at 7 days post nymph feeding (“dpf”). Spirochete burdens in (A and D) replete nymphs, (B and E) tick bite sites of skin (“Inoc. site”), and (C and F) blood were determined by qPCR. For the burdens in tissue samples, the resulting values were normalized to 100ng total DNA. Shown are the geometric means of bacterial loads ± 95% confidence interval of bacterial burdens from 5 quail tissues and blood or nymphs feeding on untreated quail (13 nymphs carrying the strains B31-5A15 or pCspA-PKo, 15 nymphs carrying the strains “Vector”, 12 nymphs carrying the strain pCspA-B31, 21 nymphs carrying the strain pCspA-ZQ1, or 17 nymphs carrying the strain pCspA-L246D), or the nymphs from OmCI-treated quail (15 nymphs carrying the strains B31-5A15, pCspA-B31, or pCspA-PKo, 8 nymphs carrying the strains “Vector”, 9 nymphs carrying the strain pCspA-L246D, 8 nymphs carrying the strain pCspA-ZQ1). Significant differences (p < 0.05, Kruskal-Wallis test with the two-stage step-up method of Benjamini, Krieger, and Yekutieli) in the spirochete burdens relative to uninfected ticks or quail tissues are indicated with an asterisk.