Figure 2.
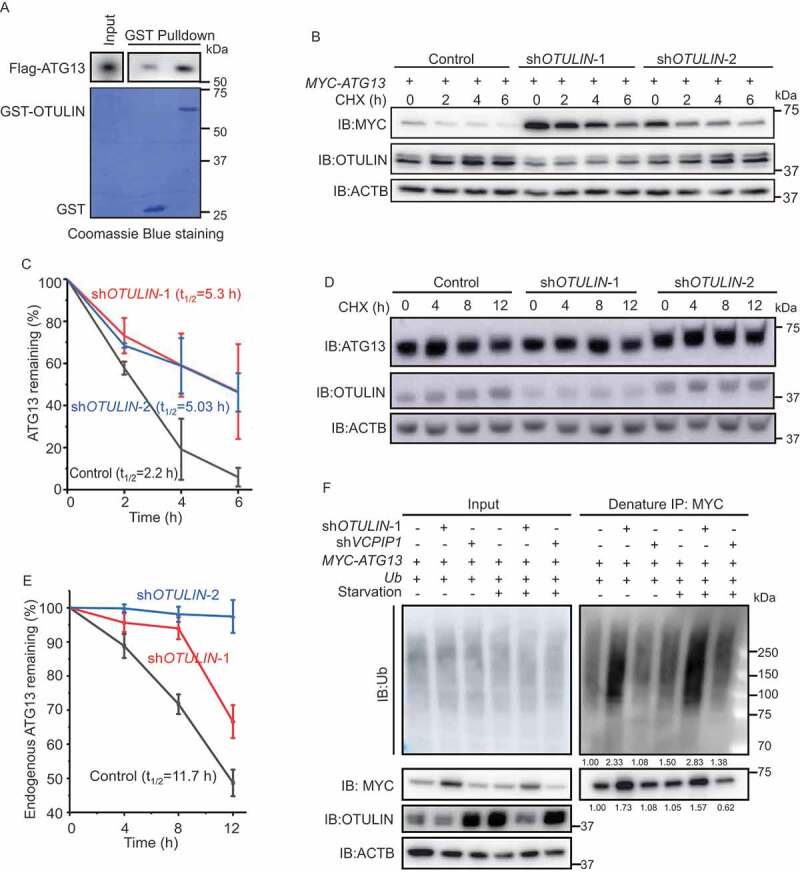
OTULIN knockdown stabilizes ATG13 protein level. (A) OTULIN binds ATG13 directly. The indicated GST or GST-OTULIN proteins were immobilized and incubated with recombinant Flag-ATG13. The precipitated proteins were analyzed by immunoblotting with an anti-Flag antibody. The Coomassie Brilliant Blue-stained gel shows the equivalent amount of GST and GST-OTULIN used for the affinity-isolation assay. (B and D) Cycloheximide (CHX) chase experiments to analyze the degradation rate of MYC-ATG13 (B) and endogenous ATG13 (D) under OTULIN knockdown conditions. Anti-MYC (B) or anti-ATG13 (D) immunoblotting was used to detect the protein level of ATG13. The degradation rate was blocked under OTULIN knockdown conditions. ACTB was used as the loading control. (C and E) Statistical analysis of the degradation rate of MYC-ATG13 or ATG13 in (B and D). Data are mean ± SD from three independent experiments. (F) Depletion of OTULIN results in accumulated ATG13 protein and ATG13-associated ubiquitin signal. HEK293 FT cells were transfected with MYC-ATG13, untagged ubiquitin, along with the control shRNA, or OTULIN shRNA-1, or the negative control VCPIP1 shRNA. Cells were treated with DMEM or EBSS for 4 h prior to lysis. Ubiquitinated proteins were immunoprecipitated with anti-MYC beads under denaturing condition and immunoblotted with an anti-ubiquitin antibody
