Figure 3.
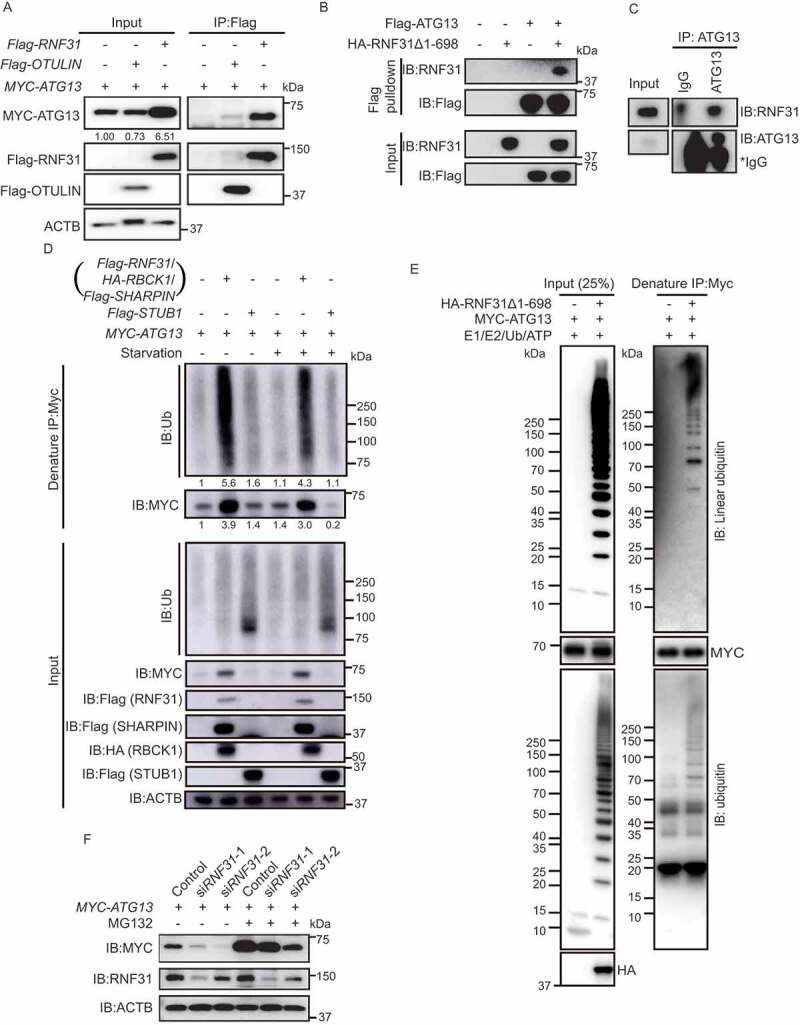
LUBAC linearly ubiquitinates ATG13 and stabilizes ATG13 protein level. (A) Flag-RNF31 and Flag-OTULIN interact with MYC-ATG13. HEK293 FT cells were transfected with MYC-ATG13 along with Flag-RNF31 or Flag-OTULIN. Flag pulldown was performed using anti-Flag beads and the samples were analyzed by immunoblotting with the indicated antibodies. (B) ATG13 interacts with RNF31 in vitro. Bacterial purified Flag-ATG13 was used to pulldown recombinant HA-RNF31Δ1-698 with anti-Flag beads. The precipitated proteins were analyzed by immunoblotting with the indicated antibodies. (C) Endogenous ATG13 interacts with RNF31 in cells. Immunoprecipitation using either IgG or anti-ATG13 was performed. The co-immunoprecipitated RNF31 was immunoblotted with an anti-RNF31 antibody. (D) MYC-ATG13 is ubiquitinated by LUBAC. HEK293 FT cells were transfected with MYC-ATG13 along with the LUBAC complex or Flag-STUB1 and were treated with DMEM or EBSS for 4 h. Ubiquitinated proteins were immunoprecipitated with anti-MYC beads under denaturing condition and immunoblotted with an anti-ubiquitin antibody. (E) In vitro ubiquitination of mammalian MYC-ATG13 by HA-RNF31Δ1-698. HEK293 FT cells were first transfected with MYC-ATG13. Immunoprecipitation of MYC-ATG13 was then performed with anti-MYC beads. The precipitated MYC beads were incubated with E1-GST, His-UBE2D2, ubiquitin, and HA-RNF31Δ1-698 for 1 h at 37°C. The reaction samples were immunoprecipitated with anti-MYC beads under denaturing conditions. Antibodies against ubiquitin and linear ubiquitin were used to detect the ubiquitination type on MYC-ATG13. (F) MYC-ATG13 protein level is decreased in RNF31 knockdown cells. HEK293 FT cells were transfected with MYC-ATG13, along with the control siRNA or RNF31 siRNA-1 or siRNA-2 and treated with DMSO or MG132 (10 μM) for 15 h. Samples were analyzed by immunoblotting with the indicated antibodies. ACTB was used as the loading control
