Abstract
Interpreting others’ beliefs, desires and intentions is known as ‘theory of mind’ (ToM), and is often evaluated using simplified measurement tools, which may not correctly reflect the brain circuits that are required for real-life ToM functioning. We aimed to identify the brain structures necessary to correctly infer intentions from realistic scenarios by administering the Awareness of Social Inference Test, Enriched subtest to 47 patients with behavioral variant frontotemporal dementia, 24 patients with progressive supranuclear palsy syndrome, 31 patients with Alzheimer’s syndrome, and 77 older healthy controls. Neuroimaging data was analyzed using voxel based morphometry, and participants’ understanding of intentions was correlated with voxel-wise and region-of interest data. We found that structural integrity of the cinguloinsular cortex in the salience network (SN) was more pivotal for accurate ToM than previously described, emphasizing the importance of the SN for selectively recognizing and attending to social cues during ToM inferences.
Keywords: Social cognition, theory of mind, neurodegenerative diseases, neuropsychology, voxel-based morphometry, salience network
1. Introduction
Research in social cognition aims to identify the mechanisms involved in perceiving, processing, and interpreting communication between oneself and others. Social cognition that relates to understanding other people’s mental states, including beliefs, desires and intentions, is often called ‘theory of mind’ (ToM) (Amodio & Frith, 2006; Gallagher & Frith, 2003). ToM is considered to be present when an individual understands that people, unlike objects, possess certain mental states that can be used to better contextualize and interpret their behavior (Lieberman, 2007). The capacity to understand and predict others’ behavior is essential for selecting an appropriate social response.
There is no unilateral theoretical framework for how ToM processes are organized cognitively. Rather, there are competing theories that can be reduced to four major accounts; the Modulatory theory, Theory theory, Simulation theory, and the Executive account, as described by Mahy, Moses, & Pfeifer (2014). According to the Modulatory account, ToM is driven by an innate neural module that is domain-specific for inferring other people’s mental states (Baron-Cohen, 1998; Leslie, Friedman, & German, 2004; Scholl & Leslie, 1999). This theory originates from findings that a set of common neural structures repeatedly activate during ToM tasks, starting from an early age. However, some studies showed that younger children do not always recruit the neural modules expected to be selective for ToM tasks (Gweon, Dodell-Feder, Bedny, & Saxe, 2012; Saxe, 2009). Additionally, tasks unrelated to ToM seem to activate ToM modules as well (Bzdok et al., 2016; Mitchell, 2008). Next, the Theory account proposes that during our life span we continuously adjust our concepts about our own and other people’s mental states based on the evidence we collect (Gopnik, 2003; Gopnik & Wellman, 2012). It proposes that we disengage from our own internal representation to shift our focus to the external, other person’s point of view. The observation that during development we adjust and advance our understanding of mental concepts, fits with the Theory theory (Wellman, Cross, & Watson, 2001); however, the neural correlates of these experience-based adjustments have been difficult to pinpoint. The third theory, the Simulation account, suggests that making mental state attributions about others relies on the ability to project one’s own mental states onto others’ mental states, i.e. to simulate what others might think or desire based on our own representations (Gallese, 2013; Goldman, 2009; Gordon, 1992). Support for this account comes from the involvement of midline structures during ToM, which are understood to play a role in self- versus other related judgements, as well as the involvement of the mirror neuron system, which is important for evaluating actions and intentions of others (Denny, Kober, Wager, & Ochsner, 2012; Gallese, 2007, 2013; Mitchell, 2009; Spreng, Mar, & Kim, 2009; Uddin, Iacoboni, Lange, & Keenan, 2007; Waytz & Mitchell, 2011). Lastly, the Executive account theorizes that the ability to attribute mental states depends on inhibition of one’s own egocentric perspective in order to accurately take the perspective of others, as well as on the ability to use working memory to keep relevant information about the other person in mind (Carlson, Claxton, & Moses, 2015; Carlson, Moses, & Breton, 2002). Studies using neural correlates to measure the importance of inhibitory control in ToM found that inhibitory circuits were necessary for adequate ToM reasoning, but not exclusive, i.e. that additional brain regions and functions also appeared necessary for ToM processing (Bull, Phillips, & Conway, 2008; Hartwright, Apperly, & Hansen, 2012).
While currently the ToM executive account primarily focuses on the regulatory processes of executive functioning, e.g. inhibition or working memory, attention processes could be important as well. In real life settings, the social cues that provide relevant information about the other person are normally derived from a continuous stream of information, thus selective attention processes might be fundamental to making correct inferences about other people’s mental states. To evaluate this possibility, selecting tasks that involve this type of realistic (i.e. continuous, multimodal) information stream would be key. However, the majority of ToM tasks are not designed to assess the importance of social component selection in realistic setting, which could be the reason why attention processes are not commonly recognized as an important contributor to ToM. For instance, the False-Belief tasks tap into inhibitory control and working memory (Drayton, Turley-Ames, & Guajardo, 2011), the Mind-in-the-Eyes test assesses recognition of facial emotions (Baron-Cohen, Wheelwright, Hill, Raste, & Plumb, 2001), and Faux-Pas stories test the understanding that one’s knowledge might be different from another person’s knowledge (Baron-cohen, Riordan, Stone, Jones, & Plaisted, 1999), but none of these tests are designed to assess the contribution of selective attention to correct ToM inference, as we expect to be required in daily life. A few ToM studies made use of more realistic ToM tasks (Kipps, Nestor, Acosta-Cabronero, Arnold, & Hodges, 2009; Shany-Ur et al., 2012), for example by testing the ability to understand other people’s intentions during sarcastic or deceitful interactions, but these studies did not systematically evaluate whether these test paradigms can support or refute the role of attention during ToM, and how well this fits with existing ToM theories.
Structural and task-based functional approaches have shown that the primary neural regions that have been identified with ToM include the dorsomedial (pregenual) prefrontal cortex, the right temporoparietal junction, and the anterior temporal lobe (Gallagher & Frith, 2003; Schurz, Radua, Aichhorn, Richlan, & Perner, 2014; Van Overwalle, 2008). Dorsomedial prefrontal cortex functions that are directly relevant to ToM are related to its role in the storage and processing of autobiographical memories, which are likely solicited when making social judgements about others that require a simulation of the social event based on previous personal experiences (Amodio & Frith, 2006; Denny et al., 2012); this is particularly relevant to the Simulation theory of ToM. The right temporoparietal junction plays a role in whether we attribute actions to another person or to ourselves. It helps us to create a sense of agency by constantly updating our internal representations based on our external experiences (Bzdok et al., 2016; Mitchell, 2008). This online cognitive awareness likely relates to some of the working memory and inhibition processes that are the focus of the Executive account of ToM, and relate to the Simulation theory by allowing us to make online self-other comparisons and distinctions (Ruby & Decety, 2001, 2003). Also, proponents of the Module theory of ToM suggest that the temporoparietal junction is such a central structure to ToM processing that it could be considered a dedicated ToM module. The anterior temporal lobe serves as memory storage for semantic information that helps one to use learned information about social conduct (Olson, Plotzker, & Ezzyat, 2007; Ross & Olson, 2010); proponents of the Theory account of ToM focus on this structure as a repository of conceptual knowledge about others that is foundational for ToM reasoning. While these three main regions are a common focus in studies, other brain structures have regularly been identified, and yet there has been less explanation posed for how they are contributing to ToM cognitive processes. For example, anterior insula involvement is often seen in ToM reasoning (Corbetta, Patel, & Shulman, 2008; Uddin & Menon, 2009; Van Der Meer, Groenewold, Nolen, & Aleman, 2011). This structure plays a central role in bringing internal representations to awareness, and helps expedite the integration of important sensory information (Craig, 2009; Seeley, Menon, et al., 2007). Involvement of the anterior insula may therefore contribute to ToM by aiding moment-by-moment identification of which stimuli in a social scene are most salient, supporting an enhanced role of online attention beyond what is emphasized in current Executive account of ToM.
These brain-behavior relationships can also be approached from a resting state functional connectivity perspective. Tight connectivity between disparate brain structures increases their efficiency when working together to accomplish a superordinate task, thus patterns of functional connectivity within intrinsically connected networks is increasingly understood to reflect efficiency of certain higher-order cognitive processes (Friston, 2011; Sutterer & Tranel, 2017). By analyzing brain-behavior relationships by grouping structures according to these known functional networks, we are not limited to understanding each structure’s independent contribution, but rather we can learn how communication among these structures influences ToM cognition. Studies using a network approach in neurotypicals and in patient groups have suggested that two intrinsically connected networks play a role in ToM processing. One of these intrinsically connected networks is the default mode network (i.e., DMN), which is related to memory manipulation processes such as the retrieval of episodic memories and comparisons among these memories to create predictions and hypothetical scenarios, and is comprised of component structures in the pregenual dorsomedial prefrontal cortex, posterior cingulate, and temporoparietal junction (Andrews-Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010; Li, Mai, & Liu, 2014; Mars et al., 2012; Raichle, 2015; Smallwood, Brown, Baird, & Schooler, 2012; Spreng et al., 2009). Based on our understanding of the cognitive functions of these DMN structures, this intrinsically connected networks likely serves ToM by enabling the generation of a mental state representation of others based on our own autobiographical memory (Andrews-Hanna, Smallwood, & Spreng, 2014; Spreng & Grady, 2010). The second network found to be related to ToM cognition is the frontoparietal network (i.e., FPN), which has component structures in the dorsolateral prefrontal, dorsolateral parietal, and precuneus cortex, and is responsible for the kind of adaptive task control commonly associated with executive functions such as working memory and other top-down control of attention (Corbetta et al., 2008; Smallwood et al., 2012).
Patients with neurodegenerative disease are a particularly important patient group for further exploration of the mechanisms by which intrinsically connected networks contribute to ToM. Seeley and colleagues (2009) established that different neurodegenerative diseases selectively affect specific intrinsically connected networks, incidentally creating a human lesion model in which the impact of single network lesion can be investigated. For example, the very earliest focal structural degeneration of patients with Alzheimer’s disease (AD) overlaps with the DMN (“memory”) network (Andrews-Hanna et al., 2010; Mars et al., 2012; Spreng et al., 2009), though additional networks are affected with disease progression, causing cognitive impairments in other domains such as language, visuospatial functioning and executive functioning (McKhann et al., 2011). When accounting for both focal DMN involvement and overall cognitive impairment, AD patients perform surprisingly well on ToM tasks. Patients show relatively poor performance only when ToM tasks are reliant on higher levels of online task control, such as working memory (e.g., the false belief task) (Bora, Walterfang, & Velakoulis, 2015). These results support the Executive account that working memory is important and perhaps even necessary for demanding forms of ToM reasoning, but do not rule out the possibility that other structures, that are not affected in mild AD, support ToM cognition as well. Furthermore, these results are paradoxical with respect to the Simulation and Theory accounts of ToM, as the pregenual dorsomedial prefrontal cortex and temporoparietal junction nodes of the DMN are important for simulation and accessing social conceptual schema in ToM; these functions are affected early in AD, but AD patients only experience limited deficits in ToM reasoning, which also suggests that structures other than the Simulation and Theory account structures are involved in ToM processes. Patients with behavioral variant frontotemporal dementia (bvFTD), and to a lesser degree progressive supranuclear palsy syndrome (PSPS), show much more substantial and pervasive ToM deficits than AD patients (Shany-Ur et al., 2012). This in itself is a challenge to all current ToM theories because bvFTD patients do not initially show damage to the brain structures most widely understood to support ToM (i.e., pregenual dorsomedial prefrontal cortex, temporoparietal junction and anterior temporal lobe), but instead show focal degeneration in the salience network (SN), an intrinsically connected network with main nodes in the anterior cingulate cortex and the ventral anterior insula that is responsible for identifying the most personally salient elements from an incoming stream of multimodal information (Seeley, Menon, et al., 2007). In PSPS, patients show altered functional connectivity in the thalamic node of the SN, which causes a disruption between cortical and subcortical connections, resulting in deficits that resemble the behavioral changes seen in bvFTD (Piattella et al., 2015; Whitwell et al., 2011). In bvFTD, degeneration of the SN results in behavioral disturbances such as disinhibition or apathy, emotion dysregulation, loss of empathy and compulsive behaviors (Rascovsky et al., 2011). According to a meta-analysis by Henry, Phillips, & Von Hippel (2014), bvFTD patients show a consistent level of poor performance across all ToM task types. In PSPS, social cognitive deficits are caused by the inability to integrate socially relevant stimuli and interpret their social meaning, which result in poor performance on ToM tasks as well (Ghosh et al., 2012). Disruption of nodes in the FPN is also common in many neurodegenerative syndromes, including a subset of patients with AD, bvFTD, and PSPS (Boeve, 2012; Perry et al., 2006; Perry & Hodges, 1999). This impacts some patients’ ability to actively maintain and manipulate information, and results in a dysexecutive behavioral pattern (Gerstenecker, Mast, Duff, Ferman, & Litvan, 2013; Stopford, Thompson, Neary, Richardson, & Snowden, 2012), which overlaps with the Executive account of ToM reasoning. While a subset of bvFTD and PSPS patients develop executive deficits such as inhibition and working memory impairment, many do not show these deficits early in their disease. Those that do have a level of executive dysfunction similar in severity to AD patients, the AD patients still perform much better on the same ToM tasks. Thus, the current version of the Executive theory of ToM does not fully account for these drastic bvFTD deficits. Another consideration is that a subset of bvFTD patients experience semantic loss (Josephs et al., 2009) perhaps more focally for socioemotional information (Ranasinghe et al., 2016). The Theory account would suggest that this would be a major cause of their ToM deficits; however, not all bvFTD patients have significant damage to this system, though all have substantial ToM impairment. This shows that existing theories (i.e., Executive-, Theory- and Simulation theory) do not satisfactorily explain the ToM deficits observed in bvFTD and PSPS patients, and suggest that additional cognitive processes and their corresponding brain systems may be responsible for these changes.
A number of these anatomic studies linking ToM functioning and brain changes in patients with neurodegenerative disease show unresolved discrepancies, thus their ability to elucidate current cognitive accounts of ToM has not yet reached its full potential. AD patients perform relatively well on ToM tasks, though the DMN –which is considered pivotal for healthy ToM (Spreng et al., 2009)– is affected early in these patients. Similarly, bvFTD and a subset of PSPS patients perform poorly on ToM tasks (Ghosh et al., 2012; Henry et al., 2014), while the intrinsically connected networks initially affected in these diseases (predominantly the SN) are not generally considered to play an important role in ToM reasoning. We propose that these seemingly paradoxical results in the ToM literature are caused by inadequate attention to the importance of salience driven attention within the Executive account of ToM. We propose that during real-life ToM cognitive processing, one must be able to adequately select and attend to relevant social information from a complex array of potential cues before one can correctly infer another’s mental state. Therefore, we chose to investigate ToM reasoning in a setting where patients with salience-driven attention deficits (i.e., bvFTD and PSPS) and healthy controls needed to infer what a person’s intentions was by watching realistic social interactions in video format. We expected that because this more ecologically valid paradigm would realistically tax the viewers’ salience attention processing (unlike Faux-Pas, the Mind-in-the-Eyes test, and other less dynamic ToM tests), it would therefore be adequate to evaluate the importance of salience driven attention in ToM reasoning. In order to show the correspondence between focal regional damage and ToM cognition, we examined the correlation of participants’ ToM response accuracy with both voxel-wise and region of interest-based structural neuroimaging data derived using voxel based morphometry to directly examine the relative contributions of the DMN, FPN and SN. We hypothesized that the ability to correctly infer someone’s intentions corresponds to volume in networks related to memory manipulation (DMN), adaptive executive control (FPN), and salience-driven attention (SN). To investigate these hypotheses, we 1) generated whole-brain voxel-wise maps of regions where brain volume linearly predicted accuracy of intention reading, and 2) performed regression modeling to compare how structural ROI volumes of each of the three functional networks predicted intention scores.
2. Methods
2.1. Participants
Data was collected from a total of 179 participants. To provide adequate inter-individual variability in both brain structure and behavior, 102 patients with neurodegenerative disease were included in the study. Of this patient group, 47 patients were diagnosed with bvFTD according to the FTD consensus criteria (Rascovsky et al., 2011), 24 with PSPS according to the Litvan criteria (Litvan et al., 1996) and 31 patients met criteria for typical AD (McKhann et al., 2011). To establish diagnosis; neuroimaging, neurological, neuropsychological and behavioral assessments were made, and patients’ diagnoses were determined by a group of multidisciplinary professionals, including neurologists, neuropsychologists and nurses. In addition, data of 77 older normal controls (ONC) was gathered. All participants were recruited for research at the Memory and Aging Center in the Department of Neurology, University of California San Francisco (UCSF). ONCs were recruited by local advertisements and recruitment talks, and were only included when no cognitive or functional deficits were found and an unremarkable neurological exam and MRI scan was established. The Committee on Human Research at UCSF approved this study, and prior to testing all participants signed an informed consent, confirming voluntary participation in research and giving permission to use the data that was collected.
2.2. Tasks and stimuli
2.2.1. Primary measure
2.2.1.1. The Awareness of Social Inference Test (TASIT) - enriched version (SI-E)
The TASIT is a clinical tool developed to assess strengths and weaknesses in social perception, focusing on comprehension of social inferences (Mcdonald, Flanagan, Rollins, & Kinch, 2003). It consists of multiple subtests, however in the present study only the information enriched (SI-E) subtest was used. This subtest of the TASIT consists out of 16 short videos, in which 8 represent a person lying to another person, and 8 represent a person being sarcastic with another person. In each video, either a visual or verbal enrichment is given to indicate the true situation. The lie vignettes entail sympathetic lies, in which the speaker is trying to keep the truth from the other person to make the best of a situation. For example, when a character’s co-worker damages his boss’ car, the character expresses in absence of him that she is concerned the boss will fire the co-worker. However, when she later talks to the co-worker, she tries to comfort him by telling him she is sure the boss will understand. The sarcastic vignettes are structurally identical to the lie scripts, but in this case the speaker is trying to emphasize, rather than conceal the truth. For instance, a character asks his wife if she thinks he has gained weight. The wife responds sarcastically by saying he looks as slim as ever, when in reality she had just mentioned in absence of her husband that she thought he had gained weight. To infer the intentions of the speaker, realistic contextual and paralinguistic cues have to be selectively attended to, and integrated from a video paradigm to create a correct understanding of a social situation, which makes this subtest an appropriate tool to measure real-life ToM based social inferences. After watching each video, four questions related to what the characters in the video do, think, say or feel assess the participants’ understanding of the displayed social interaction. The ‘do’ question best describes the participants’ ability to infer intentions from the speaker’s overall behavior, which is why only the ‘do’ question was used in this study.
2.2.2. Disease severity control measures
2.2.2.1. The CDR® Dementia Staging Instrument
The CDR is a structured interview focusing on six domains of cognitive capacity (memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care) to rate the severity of dementia (Hughes, Berg, Danziger, Coben, & Martin, 1982). Besides the global CDR score, which is calculated by using an algorithm that mainly focuses on memory, the sum of boxes score was also used, which is computed by adding up all the scores, and has the advantage that all domains are evaluated similarly (O’Bryant et al., 2008). Available patients with scores higher than 2 (out of 3) on the CDR were considered to have severe functional impairments and were therefore excluded from the study.
2.2.2.2. The Mini-Mental State Examination (MMSE)
The MMSE consists out of 30 questions related to different cognitive dimensions such as orientation, attention, recall and language, and is sensitive for picking up lower and moderate levels of impairment ((Tombaugh & McIntyre, 1992). The MMSE is mainly a language-reliant measure of disease severity. Available patients with scores lower than 11 (out of 30) on the MMSE were considered to have severe cognitive impairments and were therefore excluded from the study. In all analyses, the MMSE was used to control for cognitive impairment as a covariate of no interest.
2.2.2.3. UCSF Cognitive Theory of Mind test (cToM)
In addition to measuring disease severity by using the CDR and MMSE, we also wanted a measure identifying patients in whom their general cognitive deficits interfered with their basic ability to meaningfully attend to, track, and comprehend the social videos. The cToM shows 8 short videos in which one person changes the location of an object in the room, while the other person cannot always see that the other person had done so. 3 questions are posed after each video; the first question operates as a control question and inquires what the last seen location of the object is (“where is the object now?”), the second and third questions are respectively related to first and second order beliefs of the location of the object. The first, control question of the cToM requires a participant to pay attention to, memorize, and keep information active during the unfolding of a series of events. To control for a patient’s general cognitive capacity to successfully extract basic information from storylines by using executive, attention and memory processes, available patients with a score lower than 7 on this cToM control question total score were considered to have severe cognitive impairments and were therefore excluded from the study.
2.2.3. Structural MRI acquisition and preprocessing steps
Structural scans were obtained on a Magnetom VISION system (Siemens Inc., Iselin) and neurodegenerative patients completed the MMSE, CDR, TASIT SI-E and cToM on average 6 days before or after scanning (M=6.0, SD=14.3), which is a time-window we considered small enough to have no meaningful influence on the results. The ONCs completed the behavioral tests on average 23 days before or after scanning (M=22.7, 22.2). The healthy neurologic status was monitored and confirmed for this group, thus the time difference between testing and scanning was not anticipated to have significant influence. More information about the MRI acquisition is described in (Shany-Ur et al., 2014). Structural T1-weighted images were preprocessed using SPM12. The images were visually inspected for artifacts, and underwent bias-correction, segmentation into tissue compartments, and spatial normalization using a single generative model with the standard SPM12 parameters. The default tissue probability maps for grey matter, white matter, cerebrospinal fluid, and all other voxels from SPM12 (TPM.nii) were used (Ashburner et al., 2016). To optimize inter-subject registration, each participant’s image was warped to a template derived from 300 confirmed neurologically healthy older adults that had previously been collected at our research center (ages 44-86, M±SD: 67.2±7.3; 113 males, 186 females) scanned with one of three magnet strengths (1.5T = 27.10%; 3T = 62.88%; 4T = 10.03% of participants) using affine and nonlinear transformations with the help of the diffeomorphic anatomical registration through exponentiated lie algebra method, with standard implementation in SPM12 In all preprocessing steps, default parameters of the SPM12 toolbox were used (Ashburner et al., 2016). Total volume of each tissue compartment was calculated by applying the modulated, warped and segmented masks for gray matter, white matter, and cerebrospinal fluid to the corresponding MWS probability map for that individual, and the total intracranial volume was derived by summing the three volumes. The spatially normalized, segmented, and modulated gray matter images were smoothed with an 8-mm FWHM isotropic Gaussian kernel for use in voxel-based morphometry analysis (Acosta-Cabronero, Williams, Pereira, Pengas, & Nestor, 2007; Ashburner et al., 2016; Beagle et al., 2020; Shen & Sterr, 2013; Toller et al., 2019).
2.3. Statistical analyses
2.3.1. Behavioral data
A check for normality and violation of statistical assumptions in the primary predictors was examined using regression diagnostics. The influence of potentially confounding covariates including demographics and disease severity was evaluated using regression analyses in the Statistical Analysis System. Group differences on the control task (cToM control item total score) and group differences on the TASIT SI-E ‘do’ question were assessed with general linear models, controlling for the covariates age, sex, and global cognition (MMSE total) score. Significant pairwise differences between diagnostic groups and normal controls were assessed using post-hoc Dunnett-Hsu tests.
2.3.2. Neuroimaging data
To identify voxels in which test performance predicted gray matter volume, whole brain voxel-based morphometry analyses were conducted. For the entire sample group, one-tailed t-tests were used to examine how the TASIT SI-E ‘do’ score predicted gray matter volume. Age, sex, MMSE total, total intracranial volume and magnet strength were included as covariates. To establish the lower bound of the threshold of significance for the voxel-based morphometry analyses after family-wise error (FWE) correction, 1000 permutations of the error distribution were performed. In this approach, maximum t-values of related behavioral and imaging data were re-sampled via a Monte Carlo approach and were used to create a custom map of the error distribution of the dataset, i.e. the distribution of maximum t-values when no true relationship between the brain and behavior variables exists. The t-value at the 95th percentile of this normal error distribution was taken as the custom minimum t-threshold for results to be significant at a family-wise error corrected level of p < .05.
To examine whether volume in the intrinsically connected networks would predict the TASIT SI-E ‘do’ score, key coordinate-based functional maxima were selected from studies to define the functional hubs and extent of the DMN, FPN and SN (Andrews-Hanna et al., 2010; Beissner, Meissner, Bar, & Napadow, 2013; Dosenbach et al., 2007; Pascual et al., 2015; Seeley et al., 2009; Yeo et al., 2011). For each hub within each intrinsically connected network, a matching structural region was selected from the Neuromorphometrics atlas (Neuromorphometrics, n.d.) (see figure 1 for clarification of region selection). The volume of each intrinsically connected network was calculated for each subject by taking the average of all selected structures, and was modeled against the TASIT SI-E ‘do’ score using general linear regression analyses. First, main effect analyses were conducted, including each intrinsically connected network in a separate model to predict the TASIT SI-E ‘do’ scores. Then, influence of other networks was controlled for by including the SN and DMN in a model, the SN and FPN in a model, and the FPN and DMN in a model, each predicting the TASIT SI-E ‘do’ score. Last, the SN*DMN, SN*FPN and DMN*FPN interaction models were evaluated.
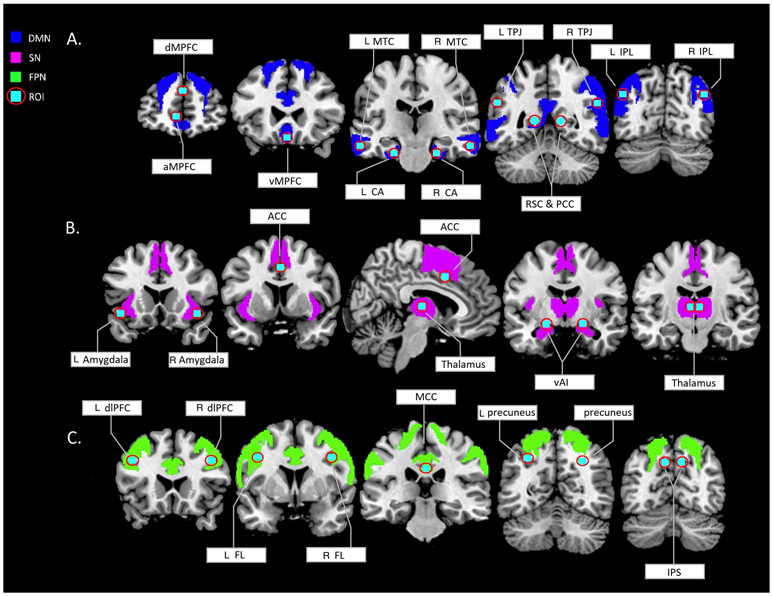
Figure 1.
regions of the neuromorphometrics map selected based on region of interests (cyan dots) from existing intrinsically connected network literature (Andrews-Hanna et al., 2010; Beissner et al., 2013; Dosenbach et al., 2007; Pascual et al., 2015; Seeley et al., 2009; Yeo et al., 2011). A. dMPFC = dorsomedial prefrontal cortex (NM = superior frontal gyrus), aMPFC = anteriomedial prefrontal cortex (NM = medial frontal cortex), vMPFC = ventromedial prefrontal cortex (NM = anterior cingulate gyrus), MTC = middle temporal cortex (NM = middle temporal gyrus), CA = hippocampal cortex (NM = parahippocampal gyrus), TPJ = temporoparietal junction (NM = middle occipital gyrus, RSC = retrosplenial cortex (NM = posterior cingulate gyrus), PCC = posterior cingulate cortex (NM = posterior cingulate gyrus), IPL = inforior perietal lobule (NM = angular gyrus). B. Amygdala (NM = amygdala), ACC = anterior cingulate cortex (NM = supplementary motor area), thalamus (NM = thalamus proper), vAI = ventral anterior insula (NM = anterior insula). C. dlPFC = dorsolateral prefrontal cortex (NM = middle frontal gyrus), FL = frontal lobule (NM = precentral gyrus), MCC = middle cingulate cortex (NM = middle cingulate gyrus), precuneus (NM = superior parietal lobule), IPS = inferior periatal sulcus (NM = supramarginal gyrus). DMN = default mode network, SN = salience network, FPN = frontoparietal network, ROI = regions of interest based on functional maxima coordinates , L = left, R = right.
3. Results
3.1. Behavioral results
All patient groups performed significantly worse than ONCs on the MMSE total, CDR global, and CDR sum of boxes (p < .001), (table 1). The average CDR scores for all diagnostic groups were below 1.2, indicating that this sample represents the earliest stages of disease progression, at a “mild cognitive impairment” or “mild dementia” level. The average age of AD and bvFTD groups was significantly lower than controls, and significantly more males were included to the bvFTD group than in to the control group. Regression analyses revealed that both the bvFTD and PSPS group performed significantly worse than the ONCs on the TASIT SI-E ‘do’ score (p < .001), (table 1).
Table 1.
Demographic information and score results of the participants (N = 179)
| M (SD) | ONC (N=77) |
AD (N=31) |
bvFTD (N=47) |
PSPS (N=24) |
F(df) | p | η2 |
|---|---|---|---|---|---|---|---|
| Age | 70.0 (7.3) | 64.3 (9.9) b | 60.0 (8.5) a | 65.7 (5.7) | 16.07 (3) | <.001 | 0.22 |
| Sex (M/F) | 31/46 | 19/12 | 30/17 b | 10/14 | 3.00 (3) | 0.03 | 0.05 |
| MMSE total (max = 30) | 29.2 (0.9) | 23.1 (4) a | 25.1 (3.1) a | 27.2 (2.2) b | 52.47 (3) | <.001 | 0.47 |
| CDR® global (0, 0.5, 1, 2) | 0 (0) | 0.8 (0.3) a | 1.2 (0.6) a | 0.7 (0.3) a | 99.51 (3) | <.001 | 0.65 |
| CDR® SOB (max = 18) | 0.02 (0.1) | 4.1 (2.3) a | 6.6 (2.8) a | 4.6 (2.4) a | 98.95 (3) | <.001 | 0.65 |
| Cognitive ToM score (Control task) ¥ (range = 7-8) | 8.0 (0.2) | 7.6 (0.5) | 7.7 (0.5) | 7.8 (0.4) | n.s. | n.s. | n.s. |
| TASIT SI-E ‘do’ score ¥ (max = 16) | 13.4 (1.9) | 11.5 (2.2) | 10.1 (2.2) a | 10.6 (2.4) a | 17.61 (3) | <.001 | 0.44 |
Note. Post hoc pair-wise comparison was performed using a Dunnett-Hsu post-hoc test, comparing each patient groups’ least squares mean with the ONC group.
Age, sex and MMSE score included as covariates for ToM and SI-E group comparisons to ONCs. AD= Alzheimer’s disease, bvFTD = behavioral variant frontotemporal dementia, PSPS = progressive supranuclear palsy syndrome, ONC = older normal controls, MMSE = mini mental state test, CDR = Dementia Staging Instrument, SOB = sum of boxes, cToM = cognitive theory of mind test, SI-E = TASIT Social Inference – Enriched test.
Group differs from ONC group at P = .001
Group differs from ONC group at P = .05
3.2. Neuroimaging results
3.2.1. Structural regions corresponding to the TASIT SI-E ‘do’ question
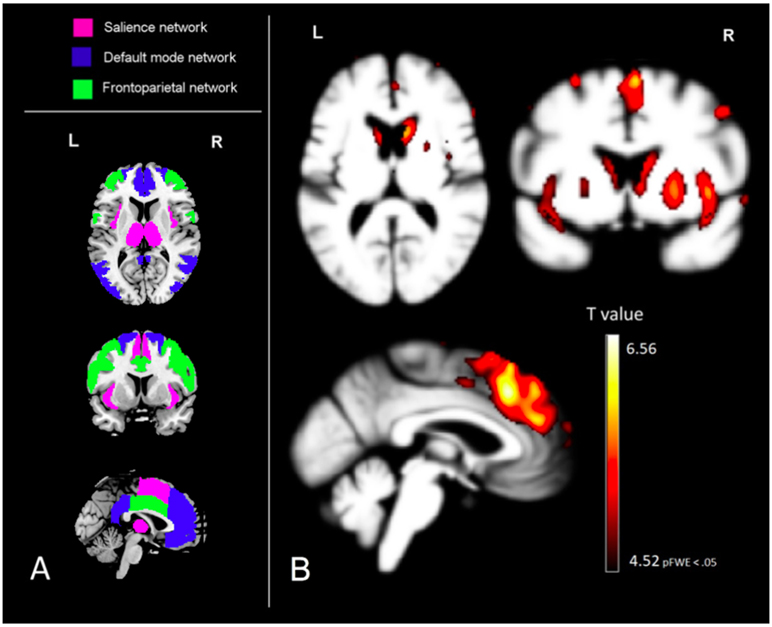
Whole brain voxel-based morphometry analysis showed that TASIT SI-E ‘do’ score significantly predicted gray matter volume in mainly frontal, temporal and subcortical areas (pFWE < .05) (figure 2b). In frontal regions, the medial prefrontal cortex (in the DMN), anterior cingulate cortex (in the SN), the middle cingulate, middle frontal and precentral gyrus (all in the FPN), alongside other frontal gray matter volumes showed above threshold correlation with the TASIT SI-E ‘do’ score. In the temporal lobes, a strong correlation was found with the insular cortex (SN) and the middle temporal gyrus and parahippocampal gyrus (DMN), alongside other temporal gray matter volumes. Other volumes in the subcortical area also predicted TASIT SI-E 'do' score, including a strong correlation with the thalamus, found in the SN (see supplementary table A).
Figure 2.
Visualization of the voxel-based morphometry derived results of the correlation between gray matter atrophy and the TASIT SI-E ‘do’ score, together with the region of interests for comparison (left). A. region of interests were derived from the Neuromorphometrics atlas. B. Voxel-wise patterns of grey matter volume in which less volume significantly predicts worse performance on the TASIT SI-E ‘do’ score; analyses controlled for age, sex, total intracranial volume, magnet strength, and MMSE score. These results are displayed at a family-wise corrected significance level of pFWE < .05, which corresponds with a minimum T value of 4.52. The same MNI coordinates are used for both image A and B; x= −1, y= 8, z= 10.
3.2.2. Relationship of volume in network related structures to the TASIT SI-E ‘do’ question
For each participant the average volume of the selected network (e.g. SN, DMN and FPN) (see table 1 and figure 2b) was modeled against the TASIT SI-E ‘do’ score. In sequential main effect analyses examining each network alone, lower volume in all three networks, i.e. the SN (b=0.0011, p<.001, CL95%=[0.00060, 0.0017]), the DMN (b=0.00020, p=0.0047, CL95%=[0.000063, 0.00034]), and the FPN (b=0.00024, p=0.0034, CL95%=[0.000081, 0.00040]), significantly predicted lower TASIT SI-E ‘do’ scores (see table 2). To ensure that the effect was not driven solely by atrophy in a single diagnostic group, we repeated these analyses controlling for the diagnostic group that was expected to have volume loss in the target network by using binary coding (dummy variables) for diagnostic group membership (Sollberger et al., 2009; Toller et al., 2019). For the bvFTD group, we expected that volume loss in the SN and FPN could drive the effects. When controlling for group membership, SN volume still significantly predicted the TASIT SI-E ‘do’ score (b=4.53, p=0.019, CL95%=[0.11, 18.92]) as well as FPN volume (b=2.8, p=0.05, CL95%=[0, 15.4]). Controlling for PSPS group membership, we expected that volume loss in the same areas as the bvFTD group could drive the effect, i.e., the SN and FPN networks. SN volume still significantly predicted the TASIT SI-E ‘do’ score (b=18.67, p<0.001, CL95%=[5.95, 41.90]), as well as FPN volume (b=7.75, p=0,0034, CL95%=[0.97, 24.60]). Controlling for AD group membership, we expected volume loss in the DMN and FPN networks. For this group, DMN volume still significantly predicted the ‘do’ score (b=4.15, p=0.024, CL95%=[0.02, 18.11]), and FPN volume still significantly predicted the ‘do’ score as well (b=5.40 p=0.012, CL95%=[0.30, 20.43]). Scatterplots showing the correlation of each network with the TASIT SI-E ‘do’ score reflect a reasonable distribution of diagnostic groups (see supplementary figure B). Together, these results suggest that frontotemporal atrophy in bvFTD patients might be driving the effect of DMN volume predicting the TASIT SI-E ‘do’ score, however for the other region of interests, these significant brain-behavior correlations seem to be generalizable across diagnostic groups.
Table 2.
Main effects of the intrinsically connected networks predicting the TASIT SI-E ‘do’ score
| ICNs included | beta | 95% confidence interval | P-value | η2 | |
|---|---|---|---|---|---|
| SN | 0.0011 | 0.00060 | 0.0017 | < 0.001 | 0.33 |
| DMN | 0.00020 | 0.000063 | 0.00034 | 0.0047 | 0.30 |
| FPN | 0.00024 | 0.000081 | 0.00040 | 0.0034 | 0.30 |
Note. Effects are controlled for age, sex and MMSE score. DMN = default mode network, FPN = frontoparietal network, SN = salience network.
When the DMN and SN volumes were included in the same model to identify their independent contributions, the SN remained significant (b=0.0022, p< .001, CL95%=[0.0010, 0.0035]), but the DMN was not significant anymore (see table 3). Similarly, when the FPN and SN were included in one model, the SN (b=0.0018, p=0.0017, CL95%=[0.00069, 0.0029]) remained significant, but the FPN was not significant anymore (see table 3). Including the DMN and FPN in the same model, both the FPN and the DMN did not significantly predict the ‘do’ score. No significant interaction effect was found between the SN and the DMN, the SN and the FPN or the FPN and DMN (see table 3).
Table 3.
Independent contributions of the intrinsically connected networks in predicting the TASIT SI-E ‘do’ score
| Networks included in the model | beta | 95% confidence interval | P-value | η2 | |
|---|---|---|---|---|---|
| DMN + SN | 0.35 | ||||
| DMN | −0.00030 | −0.00062 | 0.0000016 | n.s. | n.s. |
| SN | 0.0022 | 0.0010 | 0.0035 | <.001 | 0.049§ |
| SN*DMN | 0.000000013 | −0.00000014 | 0.00000017 | n.s. | n.s. |
| FPN + SN | 0.34 | ||||
| FPN | −0.00022 | −0.00054 | 0.00010 | n.s. | n.s. |
| SN | 0.0018 | 0.00069 | 0.0029 | 0.0017 | 0.039§ |
| SN*FPN | 0.000000011 | −0.00000013 | 0.00000016 | n.s. | n.s. |
| FPN+DMN | |||||
| FPN | 0.00020 | −0.00029 | 0.00068 | n.s. | n.s. |
| DMN | 0.000041 | −0.00038 | 0.00047 | n.s. | n.s. |
| DMN*FPN | −0.0000000020 | −0.000000050 | 0.000000045 | n.s. | n.s. |
Note. Effects are controlled for age, sex and MMSE score.
Partial η2
4. Discussion
In our study, we found that the structural integrity of the cinguloinsular cortex in the SN plays a pivotal role in understanding others’ intentions, above and beyond influences of DMN and FPN regions. This result suggests that the ability to selectively attend and respond to stimuli is an important contributor to correct ToM reasoning, and possibly for social cognition in general, especially when engaged in a naturalistic setting. Based on our results, we propose that additionally to the role of inhibitory control and working memory described in the Executive account of ToM, selective attention processes also play a significant role. This interpretation would explain how degeneration of structures that were previously not considered important for ToM affect ToM reasoning in bvFTD and PSPS patients. We also found involvement of structures typically associated with ToM, though their influence was less pronounced in our models. Overall, this demonstrates that a combination of cognitive functions are likely required for complex ToM reasoning (e.g., simulation of other’s mental states, theoretical understanding of ToM, adequate online task control), and our results contribute to these ToM theories by emphasizing the importance of salience-driven attention. Our results also lend further support to a network-interaction theory of the SN, which proposes that activation in the SN precedes activation in other networks, with the consequence that SN dysfunction directly influences the functionality of other connected networks (i.e., the DMN and FPN) (Chiong et al., 2013; Menon & Uddin, 2010).
4.1. Volume in SN structures that predict the ability to understand intentions
A key novel finding in our study is that volume in the SN, which supports attention attribution and selection of relevant stimuli (Seeley et al., 2007), strongly predicts participants’ ability to understand others’ intentions. Even in a fairly straightforward social exchange in real life, individuals are faced with many competing cues and simultaneous information from multiple modalities, thus the ability to direct attention towards the most salient stimuli could be of great benefit if one is to correctly infer other people’s intentions. However, in meta-analyses and reviews of ToM neuroanatomy, structures in the SN do not often appear to be important. This could be attributed to the type of paradigm that is used for testing ToM; predominantly tasks that are composed of complex images or videos (visual stimuli) seem to recruit the insula, whereas tasks with a more linear, unimodal or simplistically presented story, cartoon, or question format do not (Abu-Akel & Shamay Tsoory, 2011; Henry, von Hippel, Molenberghs, Lee, & Sachdev, 2015; Van Overwalle, 2008). We chose a task in which participants had to watch realistic social interactions among 2-4 speakers in video format, and then answer questions about the characters’ intentions. This demands successful integration of the words, the non-verbal prosody, the contextual cues, and other features of the scene to discern which key elements lead to a correct interpretation. As hypothesized, only patients with lesions in the SN showed impaired performance in inferring others’ intentions in this task, which implies that the SN plays an important role in ToM reasoning in complex contexts. This result sheds light on the paradoxical findings in clinical ToM literature that neurodegenerative patients with gray-matter atrophy in the structures most commonly associated with ToM (AD patients with atrophy in the DMN and FPN) show less severe ToM impairment than patients with atrophy in non-typical structures (i.e., bvFTD and PSPS) who nonetheless do have atrophy in SN structures (Bora et al., 2015; Henry et al., 2014). This emphasizes that the current theories of ToM likely do not encompass all necessary cognitive processes and thus require some revision. To incorporate selective attention processes driven by SN structures into the Executive account of ToM would considerably benefit the theoretical understanding of ToM in the research field. Our results correspond closely with findings in a study by Toller and colleagues (2018), in which individual variability in intrinsic functional connectivity in the SN correlates with differences in sensitivity to social cues in daily life. Together, this and related studies suggest that the SN may play an important role in selecting relevant social stimuli not only in ToM, but also in other aspects of social cognition, including empathy, moral reasoning, interpersonal warmth, self-regulation and emotion recognition (Betti & Aglioti, 2016; Chiong et al., 2013; Peters, Dunlop, & Downar, 2017; Shany-Ur & Rankin, 2011; Toller et al., 2019).
4.2. Volume in DMN and FPN structures that predict the ability to understand intentions
When the dominant influence of the SN is set aside, our study showed that volume in the DMN and FPN do also contribute significantly to the ability to understand others’ intentions. Our finding that DMN volume positively correlates with intention reading aligns with existing literature that suggests that a core set of regions in the DMN underlie ToM processes (Andrews-Hanna et al., 2014; Buckner, Andrews-Hanna, & Schacter, 2008; Spreng & Grady, 2010). The DMN is known to mediate memory functioning, and to make correct ToM inferences, one must often draw upon autobiographical memory to activate self-referential simulation processes, which allows one to create more accurate interpretations of social events. The significant contribution of the DMN supports the Simulation account of ToM, since these self-other comparative processes driven by the DMN are fundamental to this account. Furthermore, a meta-analysis by Spreng and colleagues (2009) showed that for both ToM and autobiographical memory tasks, better accuracy resulted from greater directional functional correspondence from the ventro- and medial prefrontal cortex through the posterior cingulate cortex and precuneus to the medial temporal lobe, as well as correspondence between the temporoparietal junction and the anterior temporal lobe. This temporoparietal junction/anterior temporal lobe connection helps retrieving personal memories and schemas of situations previously encountered and can enrich one’s understanding of the socioemotional context, which in turn may support a more complete understanding of others’ intentions (Spreng & Grady, 2010; Spreng et al., 2009). This process is an integral function of the Theory account of ToM, so this account is also supported by our results.
The FPN also plays an important role in understanding intentions. Comprehension of realistic, attentionally complex ToM tasks like the one used in our study commonly require adaptive executive attention (e.g., flexible set shifting) (Bull et al., 2008; Christoff, Gordon, Smallwood, Smith, & Schooler, 2009; Ramanan et al., 2017), which is mediated by the FPN (Dosenbach, Fair, Cohen, Schlaggar, & Petersen, 2008; Dosenbach et al., 2007; Reineberg, Andrews-Hanna, Depue, Friedman, & Banich, 2015) and are cognitive functions that are implicated in the Executive account of ToM. However, studies of healthy individuals seldom find a direct relationship between FPN activity and performance on ToM tasks (Barrett & Satpute, 2013). This perhaps occurs because healthy participants are able to function at or above the minimum threshold of executive functioning required for these ToM tasks, thus brain circuits other than the FPN more strongly predict performance. Because multiple theoretical frameworks fit with our results, our study can also be used to support the idea proposed in Schaafsma, Pfaff, Spunt & Adolphs (2015) and Schurz & Perner (2015), who suggest that there might not be a single theory that conclusively describes the structural and cognitive functions that underlie ToM, but that depending on the paradigm used, ToM processes recruit different types of structures and functions to a greater or lesser extent.
4.3. Possible modulation of salience driven attention on memory manipulation and executive control networks
The relationship of the DMN and FPN with understanding intentions statistically weakened when the SN was included in the same model. This supports the idea that even though executive functioning (FPN), and self-referential processes (DMN) may be important for understanding intentions, salience-driven attention mediated by the SN exerts a greater influence. This finding may substantiate an existing theory about how these intrinsically connected networks interact to support higher order social reasoning (Barrett & Satpute, 2013; Chiong et al., 2013; Menon & Uddin, 2010). Investigators in the domains of both autism and neurodegeneration have hypothesized that the SN can act as a gating mechanism influencing downstream processing by the DMN and the FPN. Sridharan, Levitin and Menon (2008) found that when the FPN and SN were both activated, the DMN was deactivated. More importantly, regions within the SN activated earlier than regions in the DMN and FPN, suggesting that processes related to salience distinction proceeded and mediated activation in attention or memory related networks. Chiong and colleagues (2013) used a moral reasoning functional MRI task to illustrate this same gating mechanism, finding that in healthy controls, activation of the SN predicted whether there would be downstream activation of the DMN (which preferentially engaged during personal moral reasoning) or the FPN (which was more likely to activate during impersonal reasoning). This study also found that this directional influence of the SN on downstream activation of DMN and FPN was reduced or absent in bvFTD patients, who had focal SN dysfunction and atrophy. Together, these data suggest that in real-life ToM reasoning, salience-driven attention mediated by the SN, may act as a gating mechanism that influences the function of other networks and their associated cognitive processes, depending on the degree of personal salience of the situation. When cues are detected as personally salient via healthy activation of the SN, the likelihood of DMN activation increases, and the relevant autobiographical and self-referential processes necessary for comprehensive ToM reasoning are more likely to engage. Conversely, without this characteristic co-activation of the SN-DMN that creates a milieu for autobiographically-enhanced social cognitive reasoning, the FPN is engaged by default, and complex reasoning is performed in an impersonal manner via traditional executive processes including adaptive attention and working memory. This gating mechanism could explain the poor performance of bvFTD and PSPS patients found in our study, in that SN deterioration renders complete dysfunction of downstream processes, whereas intact SN structures in AD patients still function to recruit memory and task control networks, which might be sufficient for ToM reasoning, at least early in the neurodegenerative process.
4.4. Clinical implications
The profound impact of SN integrity on patients’ ability to understand others’ intentions helps explain the ToM deficits that have been repeatedly observed in both bvFTD and PSPS patients (Shany-Ur et al., 2012). Deterioration of areas related to the SN were found to be the earliest changes underlying bvFTD (Seeley et al., 2009; Zhou et al., 2010), and changes in parts of the SN are established in patients with PSPS as well (Gardner et al., 2013; Whitwell et al., 2011). While later in the disease course many additional systems in the brain may become affected in a manner that degrades social cognition and behavior, our study highlights that focal SN damage is sufficient to impair the ability to infer other people’s intentions, and is present even at an early stage in bvFTD and some PSPS patient groups (Ghosh et al., 2012; Seeley et al., 2009). Along with loss of other forms of socioemotional sensitivity or responsiveness (Rankin et al., 2006; Toller et al., 2018, 2019), changes in an individual’s ability to infer others’ intentions should raise clinical suspicion of neurodegenerative disease in general, and perhaps bvFTD or PSPS in particular, depending on other clinical diagnostic markers.
We did not find that AD patients had clinically significant deficits in ToM functioning with our paradigm, consistent with some (Castelli et al., 2011; Gregory et al., 2002) but not all studies of ToM in AD (Ramanan et al., 2017; Verdon et al., 2007). This result further confirms the pivotal role of the SN for ToM, considering that while AD can affect memory manipulation and executive function, which are important for ToM, AD typically spares SN-related functions (Seeley, Allman, et al., 2007; Zhou et al., 2010). In our study, AD patients were very early in their disease process, which may have further reduced the influence of focal DMN and FPN damage on their ability to infer intentions.
4.5. Limitations and conclusions
While our broader goal was to gain insights into generalizable brain-behavior relationships, our study incorporated early neurodegenerative disease patients, an intentional approach designed to utilize individual differences in regional volume across participants to reveal any linear relationships between brain integrity and ToM task performance. While this approach by nature relied on structural changes to these networks, it may be considered a precursor to future studies directly investigating how functional changes in these important networks influence ToM in neurodegeneration. In functional imaging, network connectivity relies on shared synchronous activity that can be influenced by contribution of other unidentified causes, while the volumetric lesion model we used may provide somewhat stronger evidence of causality. However, one limitation of our study is that to employ this approach, we had to use structural data as a proxy to represent function in these three brain networks that are defined by their intrinsic functional connectivity. In doing this, we assume that damage to structures underpinning these networks’ functions is to some degree reflective of dysfunction in that network, i.e. that atrophy is synonymous with network dysfunction. Though our results do support and augment existing theories about how these three networks interact to support ToM processes –thus confirming that there is some validity to this assumption– additional effects related to the dynamic nature of functional connectivity may have been inadequately reflected by in our static structural brain data. Additional studies of the SN/DMN/FPN and their interactions in ToM processes will benefit from adoption of analytical methods that assess the directionality of dynamic functional activity. For example, dynamic causal modeling of functional data would yield valuable information about inter-network modulation effects that this study was not able to discriminate. Furthermore, the paradigm used in our study focused specifically on naturalistic ToM by using a video based format, which reflected the importance of selective attention processes regulated by the SN. Additional studies that focus on identifying the role of the SN during more simplistic ToM tasks could further illuminate the specific contribution of the SN to complex versus simple ToM processes.
This study demonstrated that the ability to accurately infer other people’s intentions while observing naturalistically complex social interactions requires successful engagement of attention processes that select salient cues from complex scenarios, though structural integrity of the DMN ‘memory’ and FPN ‘online task control’ networks was also contributory. We propose that our results warrant a reevaluation of the importance of salience-driven attention as part of the Executive account of ToM reasoning, in addition to the more well-described functions of inhibition and working memory. Our results also lend support to the gating hypothesis of network interaction underlying complex social cognition, which suggests that salience detection/SN function precedes and guides recruitment of other downstream networks necessary for social cognition, particularly the DMN (self-referential memory and simulation) and the FPN (adaptive executive processes). Finally, this interpretation helps disentangle previous somewhat paradoxical reports of ToM deficits in patients, and suggests that SN dysfunction may be the prime driver of ToM deficits in particular patient groups, even in the context of DMN or FPN dysfunction.
Supplementary Material
Acknowledgements
We would like to thank all the patients and caregivers for participating in this research, and Laura Bouvet for their help with data acquisition. Voxel-based morphometry analyses were performed using the UCSF Brainsight system, developed at the UCSF Memory and Aging Center by Katherine P. Rankin, Cosmo Mielke, and Paul Sukhanov, and powered by the VLSM script written by Stephen M. Wilson, with funding from the Rainwater Charitable Foundation and the UCSF Chancellor’s Fund for Precision Medicine.
Funding
This work was supported by the National Institutes of Health under Grant [numbers R01AG029577 (PI: Rankin), K23-AG021606 (PI: Rankin), P01AG019724 (PI: Miller), P50AG023501 (PI: Miller)]; Larry L. Hillblom foundation under Grant [number 2002/2j (PI: Rankin), 2014-A-004-NET (PI: Kramer)].
Footnotes
Disclosure of interest
No potential conflict of interest was reported by the authors.
References
- Abu-Akel A, & Shamay Tsoory S (2011). Neuroanatomical and neurochemical bases of theory of mind. Neuropsychologia, 49(11), 2971–2984. 10.1016/j.neuropsychologia.2011.07.012 [DOI] [PubMed] [Google Scholar]
- Acosta-Cabronero J, Williams GB, Pereira JMS, Pengas G, & Nestor PJ (2007). The impact of skull-stripping and radio-frequency bias correction on grey-matter segmentation for voxel-based morphometry. NeuroImage, 39(4), 1654–1665. 10.1016/j.neuroimage.2007.10.051 [DOI] [PubMed] [Google Scholar]
- Amodio DM, & Frith CD (2006). Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews.Neuroscience, 7(4), 268–277. 10.1038/nrn1884 [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, & Buckner RL (2010). Functional-Anatomic Fractionation of the Brain’s Default Network. Neuron, 65(4), 550–562. 10.1016/j.neuron.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, & Spreng RN (2014). The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences, 1316(1), 29–52. 10.1111/nyas.12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Barnes G, Chen C, Deaunizeau J, Flandin G, Friston K, … Penny W (2016). SPM12 manual. Retrieved from http://www.fil.ion.ucl.ac.uk/spm/doc/spm12 manual [Google Scholar]
- Baron-Cohen S (1998). Does the study of autism justify minimalist innate modularity? Learning and Individual Differences, 10(3), 179–191. 10.1016/S1041-6080(99)80129-0 [DOI] [Google Scholar]
- Baron-cohen S, Riordan MO, Stone V, Jones R, & Plaisted K (1999). A new test of social sensitivity : Detection of faux pas in normal children and children with Asperger syndrome : Journal of Autism and Developmental Disorders, 29, 407–418. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, & Plumb I (2001). The “Reading the Mind in the Eyes” Test Revised Version: A Study with Normal Adults, and Adults with Asperger Syndrome or High-functioning Autism. Journal of Child Psychology and Psychiatry, 42(2), 241–251. 10.1111/1469-7610.00715 [DOI] [PubMed] [Google Scholar]
- Barrett LF, & Satpute AB (2013). Large-scale brain networks in affective and social neuroscience: Towards an integrative functional architecture of the brain. Current Opinion in Neurobiology, 23(3), 361–372. 10.1016/j.conb.2012.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beagle AJ, Zahir A, Borzello M, Kayser AS, Hsu M, Miller BL, … Chiong W (2020). Amount and delay insensitivity during intertemporal choice in three neurodegenerative diseases reflects dorsomedial prefrontal atrophy. Cortex, 124, 54–65. 10.1016/j.cortex.2019.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beissner F, Meissner K, Bar K-J, & Napadow V (2013). The Autonomic Brain: An Activation Likelihood Estimation Meta-Analysis for Central Processing of Autonomic Function. Journal of Neuroscience, 33(25), 10503–10511. 10.1523/JNEUROSCI.1103-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betti V, & Aglioti SM (2016). Dynamic construction of the neural networks underpinning empathy for pain. Neuroscience and Biobehavioral Reviews, 63, 191–206. 10.1016/j.neubiorev.2016.02.009 [DOI] [PubMed] [Google Scholar]
- Boeve BF (2012). Progressive supranuclear palsy. Parkinsonism and Related Disorders, 18(1), 192–194. 10.1016/S1353-8020(11)70060-8 [DOI] [PubMed] [Google Scholar]
- Bora E, Walterfang M, & Velakoulis D (2015). Theory of mind in behavioural-variant frontotemporal dementia and Alzheimer’s disease: A meta-analysis. Journal of Neurology, Neurosurgery and Psychiatry, 86(7), 714–719. 10.1136/jnnp-2014-309445 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, & Schacter DL (2008). The brain’s default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124, 1–38. 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- Bull R, Phillips LH, & Conway CA (2008). The role of control functions in mentalizing: Dual-task studies of Theory of Mind and executive function. Cognition, 107(2), 663–672. 10.1016/j.cognition.2007.07.015 [DOI] [PubMed] [Google Scholar]
- Bzdok D, Langner R, Schilbach L, Jakobs O, Roski C, Caspers S, … Eickhoff SB (2016). Characterization of the temporo-parietal junction by combining data-driven parcellation, complementary connectivity analyses, and functional decoding. NeuroImage, 3(10), 973–982. 10.1016/S2215-0366(16)30284-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SM, Claxton LJ, & Moses LJ (2015). The Relation Between Executive Function and Theory of Mind is More Than Skin Deep. Journal of Cognition and Development, 16(1), 186–197. 10.1080/15248372.2013.824883 [DOI] [Google Scholar]
- Carlson SM, Moses LJ, & Breton C (2002). How Specific is the Relation between Executive Function and Theory of Mind? Contributions of Inhibitory Control and Working Memory. Infant and Child Development, (11), 73–92. 10.1002/icd [DOI] [Google Scholar]
- Castelli I, Pini A, Alberoni M, Liverta-Sempio O, Baglio F, Massaro D, … Nemni R (2011). Mapping levels of theory of mind in Alzheimer’s disease: A preliminary study. Aging and Mental Health, 15(2), 157–168. 10.1080/13607863.2010.513038 [DOI] [PubMed] [Google Scholar]
- Chiong W, Wilson SM, D’Esposito M, Kayser AS, Grossman SN, Poorzand P, … Rankin KP (2013). The salience network causally influences default mode network activity during moral reasoning. Brain, 136(6), 1929–1941. 10.1093/brain/awt066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, & Schooler JW (2009). Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences, 106(21), 8719–8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Patel G, & Shulman GL (2008). The Reorienting System of the Human Brain: From Environment to Theory of Mind. Neuron, 58(3), 306–324. 10.1016/j.neuron.2008.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig ADB (2009). How do you feel — now? The anterior insula and human awareness. Nature Reviews Neuroscience, 10. 10.1038/nrn2555 [DOI] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, & Ochsner KN (2012). A Meta-analysis of Functional Neuroimaging Studies of Self- and Other Judgments Reveals a Spatial Gradient for Mentalizing in Medial Prefrontal Cortex. Journal of Cognitive Neuroscience, 24(8), 1742–1752. 10.1162/jocn_a_00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, & Petersen SE (2008). A dual-networks architecture of top-down control. Trends in Cognitive Sciences, 12(3), 99–105. 10.1016/j.tics.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, … Petersen SE (2007). Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America, 104(26), 11073–11078. 10.1073/pnas.0704320104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayton S, Turley-Ames KJ, & Guajardo NR (2011). Counterfactual thinking and false belief: The role of executive function. Journal of Experimental Child Psychology, 108(3), 532–548. 10.1016/j.jecp.2010.09.007 [DOI] [PubMed] [Google Scholar]
- Friston KJ (2011). Functional and Effective Connectivity: A Review. Brain Connectivity, 1(1), 13–36. 10.1089/brain.2011.0008 [DOI] [PubMed] [Google Scholar]
- Gallagher HL, & Frith CD (2003). Functional imaging of ‘theory of mind.’ Trends in Cognitive Sciences, 7(2), 77–83. 10.1016/S1364-6613(02)00025-6 [DOI] [PubMed] [Google Scholar]
- Gallese V (2007). Before and below “theory of mind”: Embodied simulation and the neural correlates of social cognition. Philosophical Transactions of the Royal Society B: Biological Sciences, 362(1480), 659–669. 10.1098/rstb.2006.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V (2013). Mirror Neurons and the Simulation Theory of Mindreading. In Joint Ventures: Mindreading, Mirroring, and Embodied Cognition. 10.1093/acprof:osobl/9780199874187.003.0003 [DOI] [Google Scholar]
- Gardner RC, Boxer AL, Trujillo A, Mirsky JB, Guo CC, Gennatas ED, … Seeley WW (2013). Intrinsic connectivity network disruption in progressive supranuclear palsy. Annals of Neurology, 73(5), 603–616. 10.1002/ana.23844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstenecker A, Mast B, Duff K, Ferman TJ, & Litvan I (2013). Executive dysfunction is the primary cognitive impairment in progressive supranuclear palsy. Archives of Clinical Neuropsychology, 28(2), 104–113. 10.1093/arclin/acs098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh BCP, Calder AJ, Peers PV, Lawrence AD, Acosta-Cabronero J, Pereira JM, … Rowe JB (2012). Social cognitive deficits and their neural correlates in progressive supranuclear palsy. Brain : A Journal of Neurology, 135(7), 2089–2102. 10.1093/brain/aws128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman AI (2009). Mirroring, simulating and mindreading. Mind and Language, 24(2), 235–252. 10.1111/j.1468-0017.2008.01361.x [DOI] [Google Scholar]
- Gopnik A (2003). The theory theory as an alternative to the innateness hypothesis. In Chomsky and his critics (pp. 238–254). [Google Scholar]
- Gopnik A, & Wellman HM (2012). Reconstructing constructivism: Causal models, Bayesian learning mechanisms, and the theory theory. Psychological Bulletin, 138(6), 1085–1108. 10.1037/a0028044.Reconstructing [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon RM (1992). The Simulation Theory: Objections and Misconceptions. Mind & Language, 7(1–2), 11–34. 10.1111/j.1468-0017.1992.tb00195.x [DOI] [Google Scholar]
- Gregory C, Lough S, Stone V, Erzinclioglu S, Martin L, Simon B-C, … Hodges JR (2002). Theory of mind in patients with frontal variant frontotemporal dementia and Alzheimer’s disease: theoretical and practical implications. Brain, 125(4), 752–764. 10.1093/brain/awf079 [DOI] [PubMed] [Google Scholar]
- Gweon H, Dodell-Feder D, Bedny M, & Saxe R (2012). Theory of Mind Performance in Children Correlates With Functional Specialization of a Brain Region for Thinking About Thoughts. Child Development, 83(6), 1853–1868. 10.1111/j.1467-8624.2012.01829.x [DOI] [PubMed] [Google Scholar]
- Hartwright CE, Apperly IA, & Hansen PC (2012). Multiple roles for executive control in belief–desire reasoning: Distinct neural networks are recruited for self perspective inhibition and complexity of reasoning. NeuroImage, 61(4), 921–930. 10.1016/j.neuroimage.2012.03.012 [DOI] [PubMed] [Google Scholar]
- Henry JD, Phillips LH, & Von Hippel C (2014). A meta-analytic review of theory of mind difficulties in behavioural-variant frontotemporal dementia. Neuropsychologia, 56(1), 53–62. 10.1016/j.neuropsychologia.2013.12.024 [DOI] [PubMed] [Google Scholar]
- Henry JD, von Hippel W, Molenberghs P, Lee T, & Sachdev PS (2015). Clinical assessment of social cognitive function in neurological disorders. Nature Reviews Neurology, 12(1), 28–39. 10.1038/nrneurol.2015.229 [DOI] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben L, & Martin RL (1982). A new clinical state for the staging of dementia. British Journal of Psychiatry, 140, 566–572. 10.1192/bjp.140.6.566 [DOI] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Knopman DS, Boeve BF, Vemuri P, Senjem ML, … Jack CR (2009). Two distinct subtypes of right temporal variant frontotemporal dementia. Neurology, 73(18), 1443–1450. 10.1212/WNL.0b013e3181bf9945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipps CM, Nestor PJ, Acosta-Cabronero J, Arnold R, & Hodges JR (2009). Understanding social dysfunction in the behavioural variant of frontotemporal dementia: The role of emotion and sarcasm processing. Brain, 132(3), 592–603. 10.1093/brain/awn314 [DOI] [PubMed] [Google Scholar]
- Leslie AM, Friedman O, & German TP (2004). Core mechanisms in “theory of mind.” Trends in Cognitive Sciences, 8(12), 528–533. 10.1016/j.tics.2004.10.001 [DOI] [PubMed] [Google Scholar]
- Li W, Mai X, & Liu C (2014). The default mode network and social understanding of others: what do brain connectivity studies tell us. Frontiers in Human Neuroscience, 8, 74. 10.3389/fnhum.2014.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD (2007). Social cognitive neuroscience: a review of core processes. Annual Review of Psychology, 58, 259–289. 10.1146/annurev.psych.58.110405.085654 [DOI] [PubMed] [Google Scholar]
- Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, … Zee DS (1996). Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology, 47(1), 1–9. 10.1212/WNL.47.1.1 [DOI] [PubMed] [Google Scholar]
- Mahy CEV, Moses LJ, & Pfeifer JH (2014). How and where: Theory-of-mind in the brain. Developmental Cognitive Neuroscience. Elsevier Ltd. 10.1016/j.dcn.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Neubert F-X, Noonan MP, Sallet J, Toni I, & Rushworth MFS (2012). On the relationship between the “default mode network” and the “social brain.” Frontiers in Human Neuroscience, 6, 1–9. 10.3389/fnhum.2012.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdonald S, Flanagan S, Rollins J, & Kinch J (2003). A new clinical tool for assessing social perception after traumatic brain injury. Journal of Head Trauma Rehabilitation, 18(3), 217–238. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, … Phelps CH (2011). The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s and Dementia, 7(3), 263–269. 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, & Uddin LQ (2010). Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function, 1–13. 10.1007/s00429-010-0262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP (2008). Activity in right temporo-parietal junction is not selective for theory-of-mind. Cerebral Cortex, 18(2), 262–271. 10.1093/cercor/bhm051 [DOI] [PubMed] [Google Scholar]
- Mitchell JP (2009). Inferences about mental states. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1521), 1309–1316. 10.1098/rstb.2008.0318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuromorphometrics. (n.d.). Building a Model of the Living Human Brain. Retrieved from http://www.neuromorphometrics.com/
- O’Bryant SE, Waring SC, Cullum CM, Hall J, Lacritz L, Massman PJ, … Doody R (2008). Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: a Texas Alzheimer’s research consortium study. Archives of Neurology, 65(8), 1091–1095. 10.1001/archneur.65.8.1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, & Ezzyat Y (2007). The Enigmatic temporal pole: A review of findings on social and emotional processing. Brain, 130(7), 1718–1731. 10.1093/brain/awm052 [DOI] [PubMed] [Google Scholar]
- Pascual B, Masdeu JC, Hollenbeck M, Makris N, Insausti R, Ding SL, & Dickerson BC (2015). Large-scale brain networks of the human left temporal pole: A functional connectivity MRI study. Cerebral Cortex, 25(3), 680–702. 10.1093/cercor/bht260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RJ, Graham A, Williams G, Rosen H, Erzinçlioglu S, Weiner M, … Hodges J (2006). Patterns of frontal lobe atrophy in frontotemporal dementia: A volumetric MRI study. Dementia and Geriatric Cognitive Disorders, 22(4), 278–287. 10.1159/000095128 [DOI] [PubMed] [Google Scholar]
- Perry RJ, & Hodges JR (1999). Attention and executive deficits in Alzheimer’s disease A critical review. Brain, 122, 383–404. [DOI] [PubMed] [Google Scholar]
- Peters SK, Dunlop K, & Downar J (2017). Cortico-Striatal-Thalamic Loop Circuits of the Salience Network: A Central Pathway in Psychiatric Disease and Treatment. Frontiers in Systems Neuroscience, 10, 104. 10.3389/fnsys.2016.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piattella MC, Tona F, Bologna M, Sbardella E, Formica A, Petsas N, … Pantano P (2015). Disrupted resting-state functional connectivity in progressive supranuclear palsy. AJNR. American Journal of Neuroradiology, 36(5), 915–921. 10.3174/ajnr.A4229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME (2015). The Brain’s Default Mode Network. Annual Review of Neuroscience, 38(1), 433–447. 10.1146/annurev-neuro-071013-014030 [DOI] [PubMed] [Google Scholar]
- Ramanan S, de Souza LC, Moreau N, Sarazin M, Teixeira AL, Allen Z, … Bertoux M (2017). Determinants of theory of mind performance in Alzheimer’s disease: A data-mining study. Cortex, 88, 8–18. 10.1016/j.cortex.2016.11.014 [DOI] [PubMed] [Google Scholar]
- Ranasinghe KG, Rankin KP, Pressman PS, Perry DC, Lobach IV, Seeley WW, … Miller BL (2016). Distinct subtypes of behavioral variant frontotemporal dementia based on patterns of network degeneration. JAMA Neurology, 73(9), 1078–1088. 10.1001/jamaneurol.2016.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin KP, Gorno-Tempini ML, Allison SC, Stanley CM, Glenn S, Weiner MW, & Miller BL (2006). Structural anatomy of empathy in neurodegenerative disease. Brain, 129(11), 2945–2956. 10.1093/brain/awl254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, … Miller BL (2011). Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain, 134(9), 2456–2477. 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineberg AE, Andrews-Hanna JR, Depue BE, Friedman NP, & Banich MT (2015). Resting-state networks predict individual differences in common and specific aspects of executive function. NeuroImage, 104, 69–78. 10.1016/j.neuroimage.2014.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross LA, & Olson IR (2010). Social cognition and the anterior temporal lobes. NeuroImage, 49(4), 3452–3462. 10.1016/j.neuroimage.2009.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby P, & Decety J (2001). Effect of subjective perspective taking during simulation of action: a PET investigation of agency. Nature Neuroscience, 4(5), 546. [DOI] [PubMed] [Google Scholar]
- Ruby P, & Decety J (2003). What you believe versus what you think they believe: A neuroimaging study of conceptual perspective-taking. European Journal of Neuroscience, 17(11), 2475–2480. 10.1046/j.1460-9568.2003.02673.x [DOI] [PubMed] [Google Scholar]
- Saxe R (2009). The neural evidence for simulation is weaker than i think you think it is. Philosophical Studies, 144(3), 447–456. 10.1007/s11098-009-9353-2 [DOI] [Google Scholar]
- Schaafsma SM, Pfaff DW, Spunt RP, & Adolphs R (2015). Deconstructing and reconstructing theory of mind. Trends in Cognitive Sciences. NIH Public Access. 10.1016/j.tics.2014.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl BJ, & Leslie AM (1999). Modularity, development and “theory of mind.” Mind and Language, 14(1), 131–153. 10.1111/1468-0017.00106 [DOI] [Google Scholar]
- Schurz M, & Perner J (2015). An evaluation of neurocognitive models of theory of mind. Frontiers in Psychology. Frontiers Research Foundation. 10.3389/fpsyg.2015.01610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurz M, Radua J, Aichhorn M, Richlan F, & Perner J (2014). Fractionating theory of mind: A meta-analysis of functional brain imaging studies. Neuroscience and Biobehavioral Reviews, 42, 9–34. 10.1016/j.neubiorev.2014.01.009 [DOI] [PubMed] [Google Scholar]
- Seeley WW, Allman JM, Carlin DA, Crawford RK, Macedo MN, Greicius MD, … Miller BL (2007). Divergent Social Functioning in Behavioral Variant Frontotemporal Dementia and Alzheimer Disease : Reciprocal Networks and Neuronal Evolution. Alzheimer Disease and Associated Disorders, 21(4), 50–57. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, & Greicius MD (2009). Neurodegenerative Diseases Target Large-Scale Human Brain Networks. Neuron, 62(1), 42–52. 10.1016/j.neuron.2009.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, … Greicius MD (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci, 27(9), 2349–2356. 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shany-Ur T, Lin N, Rosen HJ, Sollberger M, Miller BL, & Rankin KP (2014). Self-awareness in neurodegenerative disease relies on neural structures mediating reward-driven attention. Brain, 137(8), 2368–2381. 10.1093/brain/awu161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shany-Ur T, Poorzand P, Grossman SN, Growdon ME, Jang JY, Ketelle RS, … Rankin KP (2012). Comprehension of insincere communication in neurodegenerative disease: Lies, sarcasm, and theory of mind. Cortex, 48(10), 1329–1341. 10.1016/j.cortex.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shany-Ur T, & Rankin KP (2011). Personality and social cognition in neurodegenerative disease. Current Opinion in Neurology, 24(6), 550–555. 10.1097/WCO.0b013e32834cd42a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, & Sterr A (2013). Is DARTEL-based voxel-based morphometry affected by width of smoothing kernel and group size? A study using simulated atrophy. Journal of Magnetic Resonance Imaging, 37(6), 1468–1475. 10.1002/jmri.23927 [DOI] [PubMed] [Google Scholar]
- Smallwood J, Brown K, Baird B, & Schooler JW (2012). Cooperation between the default mode network and the frontal-parietal network in the production of an internal train of thought. Brain Research, 1428, 60–70. 10.1016/j.brainres.2011.03.072 [DOI] [PubMed] [Google Scholar]
- Sollberger M, Stanley CM, Wilson SM, Gyurak A, Beckman V, Growdon M, … Rankin KP (2009). Neural basis of interpersonal traits in neurodegenerative diseases. Neuropsychologia, 47(13), 2812–2827. 10.1016/j.neuropsychologia.2009.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, & Grady CL (2010). Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. Journal of Cognitive Neuroscience, 22(6), 1112–1123. 10.1162/jocn.2009.21282 [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, & Kim ASN (2009). The Common Neural Basis of Autobiographical Memory, Prospection, Navigation, Theory of Mind, and the Default Mode: A Quantitative Meta-analysis. Journal of Cognitive Neuroscience, 21(3), 489–510. 10.1162/jocn.2008.21029 [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, & Menon V (2008). A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Networks, 105(34), 12569–12574. 10.1073/pnas.0800005105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopford CL, Thompson JC, Neary D, Richardson AMT, & Snowden JS (2012). Working memory, attention, and executive function in Alzheimer’s disease and frontotemporal dementia. Cortex, 48(4), 429–446. 10.1016/j.cortex.2010.12.002 [DOI] [PubMed] [Google Scholar]
- Sutterer MJ, & Tranel D (2017). Neuropsychology and cognitive neuroscience in the fMRI era. Neuropsychology, 31(8), 972–980. 10.1037/neu0000408.Neuropsychology [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toller G, Brown J, Sollberger M, Shdo SM, Bouvet L, Sukhanov P, … Rankin KP (2018). Individual differences in socioemotional sensitivity are an index of salience network function. Cortex, 103, 211–223. 10.1016/j.cortex.2018.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toller G, Yang WFZ, Brown JA, Ranasinghe KG, Shdo SM, Kramer JH, … Rankin KP (2019). Divergent patterns of loss of interpersonal warmth in frontotemporal dementia syndromes are predicted by altered intrinsic network connectivity. NeuroImage: Clinical, 22, 101729. 10.1016/j.nicl.2019.101729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh TN, & McIntyre NJ (1992). The mini-mental state examination: a comprehensive review. Journal of the American Geriatrics Society, 40(9), 922–935. 10.1111/j.1532-5415.1992.tb01992.x [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Iacoboni M, Lange C, & Keenan JP (2007). The self and social cognition: the role of cortical midline structures and mirror neurons. Trends in Cognitive Sciences, 11(4), 153–157. 10.1016/j.tics.2007.01.001 [DOI] [PubMed] [Google Scholar]
- Uddin LQ, & Menon V (2009). The anterior insula in autism: Under-connected and underexamined. Neuroscience and Biobehavioral Reviews, 33(8), 1189–1203. 10.1177/004057360506200212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Meer L, Groenewold NA, Nolen WA, & Aleman A (2011). Inhibit yourself and understand the other: Neural basis of distinct processes underlying Theory of Mind. NeuroImage. 10.1016/j.neuroimage.2011.03.053 [DOI] [PubMed] [Google Scholar]
- Van Overwalle F (2008). Social cognition and the brain: A meta-analysis. Human Brain Mapping, 30(3), 829–858. 10.1002/hbm.20547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdon CM, Fossati P, Verny M, Dieudonné B, Teillet L, & Nadel J (2007). Social cognition: An early impairment in dementia of the Alzheimer type. Alzheimer Disease and Associated Disorders, 21(1), 25–30. 10.1097/WAD.0b013e318032487a [DOI] [PubMed] [Google Scholar]
- Waytz A, & Mitchell JP (2011). Two mechanisms for simulating other minds: Dissociations between mirroring and self-projection. Current Directions in Psychological Science, 20(3), 197–200. 10.1177/0963721411409007 [DOI] [Google Scholar]
- Wellman HM, Cross D, & Watson J (2001). Meta-analysis of theory-of-mind development: The truth about false belief. Child Development, 72(3), 655–684. 10.1111/1467-8624.00304 [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Avula R, Master A, Vemuri P, Senjem ML, Jones DT, … Josephs KA (2011). Disrupted thalamocortical connectivity in PSP: A resting-state fMRI, DTI, and VBM study. Parkinsonism and Related Disorders, 17(8), 599–605. 10.1016/j.parkreldis.2011.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, … Buckner RL (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol, 106(3), 1125–1165. 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, … Seeley WW (2010). Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain, 133(5), 1352–1367. 10.1093/brain/awq075 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.