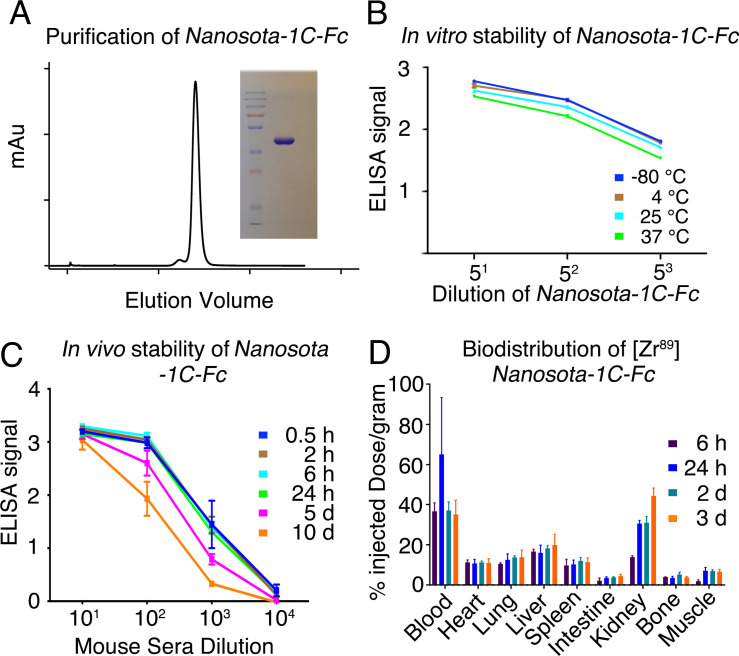
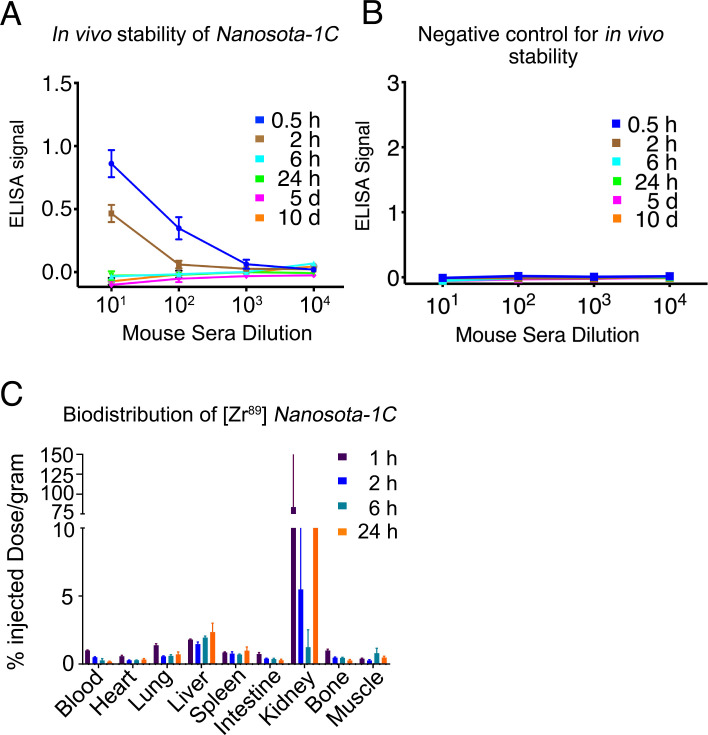
Figure 5. Analysis of expression, purification, and pharmacokinetics of Nanosota-1C-Fc.
(A) Purification of Nanosota-1C-Fc from bacteria. The protein was nearly 100% pure after gel filtration chromatography, as demonstrated by its elution profile and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (stained by Coomassie blue). The yield of the protein was 40 mg/l of bacterial culture, without any optimization of the expression. (B) In vitro stability of Nanosota-1C-Fc. The protein was stored at indicated temperatures for a week, and then a dilution enzyme-linked immunosorbent assay (ELISA) was performed to evaluate its SARS-CoV-2 receptor-binding domain (RBD)-binding capability. Data are the mean ± SEM (n = 4). (C) In vivo stability of Nanosota-1C-Fc. Nanosota-1C-Fc was injected into mice, mouse sera were collected at various time points, and Nanosota-1C-Fc remaining in the sera was detected for its SARS-CoV-2 RBD-binding capability as displayed in a dilution ELISA. Data are the mean ± SEM (n = 3). (D) Biodistribution of [89Zr]Zr-Nanosota-1C-Fc. Nanosota-1C-Fc was radioactively labeled with zirconium-89 (89Zr) and injected into mice via the tail vein. Different tissues or organs were collected at various time points (n = 3 mice per time point). The amount of Nanosota-1C-Fc present in each tissue or organ was measured through examining the radioactive count of each tissue or organ. Data are the mean ± SEM (n = 3).