Abstract
Arterial blood gas (ABG) measurements at both maximum depth and at resurfacing prior to breathing have not previously been measured during free dives conducted to extreme depth in cold open-water conditions. An elite free diver was instrumented with a left radial arterial cannula connected to two sampling syringes through a low-volume splitting device. He performed two open-water dives to a depth of 60 m (197′, 7 atmospheres absolute pressure) in the constant weight with fins competition format. ABG samples were drawn at 60 m (by a mixed-gas scuba diver) and again on resurfacing before breathing. An immersed surface static apnea, of identical length to the dives and with ABG sampling at identical times, was also performed. Both dives lasted approximately 2 min. Arterial partial pressure of oxygen () increased during descent from an indicative baseline of 15.8 kPa (after hyperventilation and glossopharyngeal insufflation) to 42.8 and 33.3 kPa (dives 1 and 2) and decreased precipitously (to 8.2 and 8.6 kPa) during ascent. Arterial partial pressure of carbon dioxide () also increased from a low indicative baseline of 2.8 kPa to 6.3 and 5.1 kPa on dives 1 and 2; an increase not explained by metabolic production of CO2 alone since actually decreased during ascent (to 5.2 and 4.5 kPa). Surface static apnea caused a steady decrease in and increase in without the inflections provoked by depth changes. Lung compression and expansion provoke significant changes in both and during rapid descent and ascent on a deep free dive. These changes generally support predictive hypotheses and previous findings in less extreme settings.
NEW & NOTEWORTHY Arterial blood gas measurements at both maximum depth and the surface before breathing on the same dive have not previously been obtained during deep breath-hold dives in cold open-water conditions and competition dive format. Such measurements were obtained in two dives to 60 m (197′) of 2 min duration. Changes in arterial oxygen and carbon dioxide (an increase during descent, and a decrease during ascent) support previous observations in less extreme dives and environments.
Keywords: apnea, carbon dioxide, free diving, glossopharyngeal insufflation, oxygen
INTRODUCTION
Free diving (breath-hold diving) is undertaken for activities such as underwater tourism and food gathering and as a competitive sport. Free diving requires apnea during submersion, and safe conduct, therefore, relies on return to the surface and resumption of breathing before the onset of critical hypoxia, which may lead to loss of consciousness and drowning. Such events were considered the disabling injury in 12% of 175 Australian snorkel diving fatalities (1). Hypoxic events are common in free diving competitions. In 344 constant weight performances (swimming down and up with fins and no other assistance, maintaining the same weight profile throughout), there was hypoxic loss of motor control in 6.1% and loss of consciousness (often referred to by free divers as “shallow-water blackout”) in a further 6.1% (2).
A long-standing paradigm for patterns of change in arterial oxygen (O2) and carbon dioxide (CO2) in free diving holds that despite apnea, compression of the lungs and gas contained therein by increasing water pressure during descent would actually increase the alveolar and arterial pressures of O2 ( and , respectively) while having little effect on arterial pressure of CO2 () because of its markedly greater solubility in blood and tissue. Conversely, after continued consumption of O2 during the deeper phase of the dive, there would be a precipitous fall in and as the lungs expand during ascent, rendering the diver prone to loss of consciousness as they approach the surface (3, 4). The increase in at depth coupled with the relatively small increase in (which many divers lower before diving by hyperventilating) could blunt respiratory drive at depth and encourage the diver to dangerously prolong their dive.
At least some aspects of this paradigm have been validated in three human studies using arterial blood gas (ABG) sampling (5–7). However, these studies were all conducted under circumstances that complicate extrapolation of results to real-world extreme free diving. One was undertaken in the wet compartment of a hyperbaric chamber in relatively warm water (25°C) with slow (1 min) transitions to and from a pressure equivalent to only 20 meters of seawater (msw) (4). Two studies involved free diving in a warm water pool (31°C) to 40 meters of fresh water (mfw) with ABG specimens taken either at depth (6) or at the surface before breathing (7) but not both on the same dive.
The present study extended this previous work by taking ABG specimens from an elite free diver both at maximum depth and (in the same dive) at the surface before breathing, in dives to a much more challenging depth [60 mfw, 197′, 7 atmospheres absolute (ATA)] and in open-water environmental conditions similar to a real-world free diving competition. The relatively simple aim of this complex undertaking was to compare the resulting patterns of change in and to those broadly predicted by the physiological paradigm articulated above and to those obtained by other studies conducted in much less extreme conditions (5–7).
MATERIALS AND METHODS
The study protocol was approved by the Health and Disability Ethics Committees of the New Zealand Ministry of Health, Ref. No. 20/NTB/218. The deep free diver consented to publication of identifying information and a photograph, as documented in the appropriate form.
Free Diver Subject
The free diver subject (male, 39 yr) provided written informed consent for participation. He is a former world record holder in both dynamic apnea categories (swimming horizontally in a swimming pool with or without fins; 265 and 218 m distance, respectively) and a former world champion in the constant weight with fins category [126 msw (413′) depth]. He had undergone body plethysmography at the time of his dynamic apnea records in 2007 and this was not repeated. For the present field study, he wore an unlined 5-mm Heiwa high-density neoprene top and 2-mm waist-high pants (Elios Sub Wetsuits, Cattolica, Italy), homemade fluid-filled goggles, a free diving nose clip (Trygons, Athens, Greece), a Suunto D4 dive watch (Suunto, Vantaa, Finland), and a Molchanovs S2 monofin (Mochanovs, https://molchanovs.com). He was loosely connected to the shot line by a 1-m safety lanyard. He was neutrally buoyant at 12 mfw.
Setting and Team Logistics
The study took place at Lake Taupo [altitude 356 m (1,168′)], New Zealand. The water temperature was 18°C at the surface and 10°C at 60 mfw depth with a thermocline at 15 mfw depth. A competition free diving rig was deployed on an inflatable platform next to an anchored boat. This rig provided a vertical weighted line to the target depth with a “counter-ballast” system whose operation would result in the line and the free diver being rapidly retrieved to the surface in the event he did not return when expected. A second nonanchored boat was present for emergency evacuation from the dive site if necessary.
The team included two mixed-gas (O2, helium, nitrogen) closed-circuit rebreather divers (one an anesthetist) who dived to 60 mfw for 36 min to take the deep ABG specimens and, thereafter, completing a 2-h decompression. In accordance with free diving competition guidelines, there were two safety free divers; one who met the ascending subject at 30 mfw, and the other at 15 mfw. Thus, the last part of each ascent was accompanied by two safety free divers. Another anesthetist team member was immersed at the surface to take the surface ABG specimens before the free diver resumed breathing. Two team members had trained on the i-Stat Alinity point-of-care blood gas analyzer (Abbott Diagnostics, Princeton, NJ, RRID:SCR_008392) to facilitate the processing of specimens immediately on the boat when the free diver reached the surface.
Arterial Blood Gas Specimen Collection
Flow in the radial and ulnar arteries was checked using color-flow ultrasound (Butterfly iQ, Guildford, CT). After shaving the skin, the wet suit was donned and the skin prepared with 2% chlorhexidine in alcohol. An anesthetist team member inserted a 48-mm 20-g BD Insyte cannula (Becton Dickinson, Franklin Lakes, NJ, RRID:SCR_008418) into the free diver’s nondominant (left) radial artery under local anesthesia (2% lidocaine) and ultrasound guidance. The cannula was connected to a bespoke low-volume (0.5-mL dead space) splitting device incorporating thick-walled narrow-bore tubing and isolation clips that allowed simultaneous connection of two 5-mL syringes (Fig. 1). Both the splitting device and the syringes were primed with heparinized saline solution (4 U·mL−1). The surrounding skin was widely painted with benzoin compound tincture (Humco, Austin, TX) to enhance adherence, and a Tegaderm IV dressing (3 M Deutschland, Neuss, Germany) was used to fasten the cannula to skin. This was reinforced with Sleek waterproof tape (BSN medical GmbH, Hamburg, Germany). The wet suit sleeve was folded down to the insertion site, and the syringes were taped to the wet suit using Sleek tape. Small (0.5 mL) aliquots of heparinized saline were administered ∼15 minutely during the predive and between-dive periods to ensure catheter patency. At the point of leaving surface on each of the two 60-m free dives, the syringes each contained 0.5 mL of heparinized saline. Specimen collection involved undoing the isolation clip, injecting the residual 0.5 mL of heparinized saline to ensure catheter patency, and then aspirating arterial blood to the full extent of the syringe (5.6 mL volume) before refastening the isolation clip.
Figure 1.

The arterial line, low-volume splitter, and two 5-mL syringes in place on the left (nondominant) arm of the subject. Both syringes contain heparinized saline (4 U·ml−1), and the higher-volume (medial) syringe was used to administer periodic 0.5-mL aliquots to ensure catheter patency. Prior to diving, the volume in each syringe was reduced to 0.5 mL, which was injected to clear the cannula just prior to aspirating the specimen.
Study Procedure
A complete practice run, including a 60-mfw (7 ATA) dive with simulated arterial blood sampling (but without arterial cannulation), was undertaken over 1 day. The study dives took place the following day.
Following insertion of the arterial catheter, positioning of the boat, and deployment of the free diving shot line to 60 mfw, a nonimmersed, resting ABG specimen was taken and processed. The free diving team entered the water and another ABG specimen was taken after the deep free diver’s standardized predive respiratory routine. The latter consisted of 2 min of quiet focused breathing with the last 40 s spent taking six very deep breaths, followed by 10 s of lung “packing” comprising 15 glossopharyngeal insufflation maneuvers (4). No actual dive was conducted after this first “simulated” predive respiratory routine because of the interruption associated with taking an ABG specimen and removing and replacing the syringe before diving.
The mixed-gas rebreather divers then descended and signaled their readiness at 60 mfw with pulls on the shot line. In the meantime, the free diver completed three brief “warm-up” dives to 15, 20, and 20 mfw; all starting at functional residual capacity. Ten minutes after the last of these dives, he again completed his predive respiratory routine (see previous paragraph) and descended to 60 mfw (7 ATA), where the anesthetist rebreather diver drew an ABG specimen (see Arterial Blood Gas Specimen Collection) (Fig. 2). The free diver ascended to the surface where the immersed surface anesthetist drew the second ABG specimen before breathing resumed. The syringes were immediately removed for specimen processing and replaced with syringes for the next dive. Both specimens were processed within 5 min of the first being taken. The dive itself followed the procedures and rules that would be applied in the real-world competition “constant weight” category (8).
Figure 2.
Mixed-gas diver taking an arterial blood gas specimen at 60 meters of fresh water (197′, 7 atmospheres absolute) using one of two syringes attached to the low-volume splitter.
Between dives, the free diver left the water for ∼10 min to warm up. The second 60-mfw dive was conducted in an identical manner to the abovementioned but with only one work-up dive (from functional residual capacity) to 13 mfw completed 5 min before the second deep dive. On completion of the second deep free dive, there was another period out of the water to warm up, after which the free diver reentered the water and performed a surface static apnea (breath-holding at rest face down in water) of exactly the same duration as the first deep dive. ABG specimens were taken at time points aligned precisely with the deep and surface specimens on the deep dives. Once again, the second specimen was taken before breathing resumed.
RESULTS
Free Diver Subject
The diver is a tall (197 cm), slim (96 kg, body mass index = 24.6 kg·m−2), Caucasian male with supranormal lung volumes. Body plethysmography in 2007 revealed total lung capacity of 11.94 L (138% predicted), vital capacity of 10.42 (157%), functional residual capacity of 6.27 (167%), and residual volume of 1.52 (79%) [National Health and Nutrition Examination Survey III/Economic Research Service 93 (NHANES III/ERS 93) predictive values]. Although 13 years old, based on predictive models and measured longitudinal change in diver groups (9), these values would be expected to have changed very little and the measurements were not repeated.
Dives and Arterial Blood Gas Results
The 60-mfw free dives, associated safety free dives, and the decompression from the mixed-gas dives were all undertaken without complication. The two 60-mfw free dives were virtually identical in timing, with descent = 55 s, bottom time = 13 s, ascent = 45 s, total = 113 s.
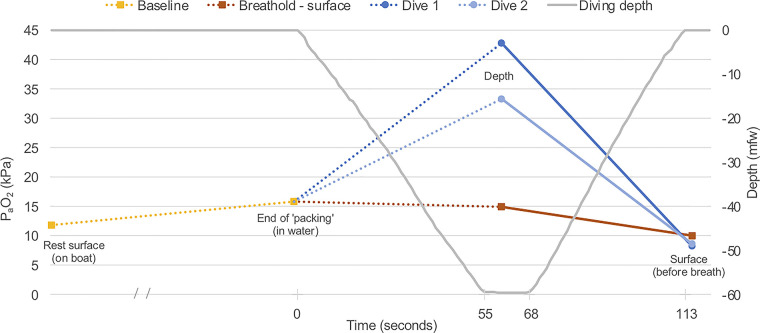
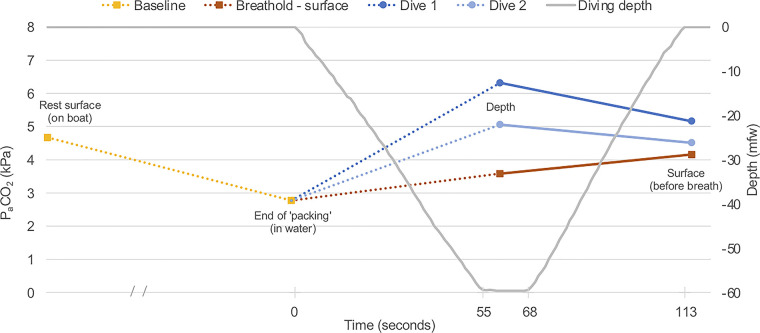
The ABG measurements at resting baseline, post-predive respiratory routine (referred to as “end of packing”), 60 mfw depth, and the surface before breathing in the two deep free dives and the identically timed specimens during the surface apnea are depicted in Fig. 3 () and Fig. 4 (). To be clear, as described in materials and methods, resting baseline and “end of packing” specimens were not taken immediately before each apnea event. The single values depicted (in the case of “end of packing,” following a standardized routine) are, therefore, indicative baselines for the two free dives and surface static apnea, reflected in the figures by the use of dotted lines linking the baselines to the dive and static apnea data.
Figure 3.
at indicative baselines (resting nonimmersed and postpredive respiratory routine—referred to as “end of packing”) and the specimens taken during the two dives and at equivalent times during static surface apnea. Use of dotted lines represents an acknowledgment that the baseline measurements were indicative (not immediately before each dive or the static surface apnea). The solid lines link measurements made on the same dive and during surface apnea. , arterial partial pressure of oxygen.
Figure 4.
at indicative baselines (resting nonimmersed and postpredive respiratory routine—referred to as “end of packing”) and the specimens taken during the two dives and at equivalent times during static surface apnea. Use of dotted lines represents an acknowledgment that the baseline measurements were indicative (not immediately before each dive or the static surface apnea). The solid lines link measurements made on the same dive and during surface apnea. , arterial partial pressure of carbon dioxide.
DISCUSSION
This study reports, for the first time, to our knowledge, ABG data from both maximum depth and at the surface (before breathing) during free dives conducted to extreme depth in cold open-water conditions and competition format. It builds on previous work to much shallower depths (in a hyperbaric chamber) (5) and where ABG specimens were taken either at depth or the surface before breathing in separate dives but not both on the same dive (6, 7).
Predive Packing Routine
There was a substantial increase in [from 11.8 to 15.8 kPa (88.5 to 118.5 mmHg)] and a fall in [from 4.7 to 2.8 kPa (35.3 to 21.0 mmHg)] between the specimens drawn during nonimmersed rest and after immersed predive deep breathing and “packing” (glossopharyngeal insufflation as described in Study Procedure). These are single observations in a single diver but are similar to those from two divers who had ABG samples taken following predive routines including packing (5). The hypocapnia is clearly explained by hyperventilation, and the reduced fraction of CO2 in alveolar gas would contribute to the rise in . Other potential contributors to the latter are consequences of glossopharyngeal insufflation including improved ventilation-perfusion matching through abolition of atelectasis and increased transpulmonary pressures that would increase the partial pressure of any gas present in the lung. Transpulmonary pressure can rise ∼2−4 kPa (15–30 mmHg) above the expected values at total lung capacity after glossopharyngeal insufflation (10).
Diving—Arterial Oxygen
The increased substantially from the indicative baseline [15.8 kPa (118.5 mmHg) at the end of the standardized predive routine] to the maximum depth [42.8 and 33.3 kPa (321.0 and 249.8 mmHg) on dives 1 and 2, respectively]. In contrast, an ABG specimen taken at exactly the same time point (55 s) during static apnea showed a small decline in [to 14.9 kPa (111.8 mmHg)] (Fig. 3). The vast increase with depth was confluent with previous findings (5, 6) and confirms the prediction that despite apnea and ongoing O2 metabolism, a free diver’s can increase during descent due to lung compression provided the latter is fast enough. One caveat to this generalization is the previous report that two of six subjects descending to 40 mfw did not exhibit such an increase (6). This was hypothesized to be attributable to atelectasis caused by lung compression, with a consequent increase in venous admixture. The difference in peak readings seen in the present study might also be an early sign of such a process if, despite identical predive respiratory routines, the starting lung volume was smaller on the second dive, for example, due to the longer total period of prior head out immersion that preceded that dive. In this setting, a greater degree of compression-induced atelectasis could increase venous admixture and depress the peak measurement at the deepest depth. The diver was also subjectively colder before the second dive and perhaps consuming more oxygen for thermogenesis.
As predicted, the fell precipitously during ascent, reaching 8.2 and 8.6 kPa (61.5 and 64.5 mmHg) at the surface (before breathing) on dives 1 and 2, respectively. Surfacing with a barely in the hypoxic range is not surprising given the dive was well inside the performance boundaries for this diver. However, the simple demonstration of such a precipitous fall between maximum depth and surface specimens taken on the same dive is a clear (and unique) physiological validation of the hazards associated with the shallow phase of an extreme free dive ascent. It is obvious how a deeper dive and/or a longer period at depth with greater consumption of alveolar O2 before ascent could result in hypoxic loss of consciousness as the plummets late in the ascent.
Diving—Arterial Carbon Dioxide
The also increased from the indicative baseline [2.8 kPa (21.0 mmHg)] to the maximum depth [6.3 and 5.1 kPa (47.3 and 38.3 mmHg) on dives 1 and 2, respectively] over a descent lasting less than 1 min. A much smaller increase [to 3.6 kPa (27.0 mmHg)] occurred in the same period during static apnea at the surface, and this is more confluent with (albeit slightly lower than) the rate of increase previously demonstrated in anesthetized humans over the first minute of apnea (11). The dives involved more exercise and greater production of CO2 would be expected, but the subsequent fall in during ascent (which can be considered “real” because of depth and surface sampling on the same dive) proves that the initial increase during descent was not driven solely by metabolic production of CO2 or development of a CO2 pressure gradient from alveoli to blood, which can occur when lung volume decreases as oxygen is consumed during apnea under some static pressure conditions (12). Our findings suggest that rapid lung compression during a fast descent can increase the alveolar Pco2 with a consequent rise in that is partly, but not completely, buffered by carbon dioxide’s extremely high solubility in tissues and blood. This phenomenon has been subtly demonstrated and discussed previously (3, 5), but the present data provide the clearest demonstration to date.
Strengths and Limitations
The most important strengths of this study are its unique ecological validity in relation to real-world extreme free diving and related competitions, and the taking of ABG specimens both at depth and at the surface before breathing on the same dive. However, there are important limitations. Most obviously, it involved only one subject and two dives to a single depth, which means that any generalization must be made cautiously. In relation to methodology, there was potential for small errors in absolute ABG values resulting from the small dead space in the arterial line splitter system being aspirated into the samples. Specimen collection from an arterial catheter typically involves aspirating and discarding any fluid or blood from system dead space before sampling. This is impractical where two specimens are to be taken on the same deep free dive because it would take too long and would require a complicated bulky system of three-way taps and syringes. Consequently, the very low dead space splitter was combined with two 5-mL sampling syringes (larger than usually used for ABG sampling) aspirated to their full volume (5.6 mL) during sampling. The potential for the 0.5-mL dead space crystalloid fluid to affect the ABG result from a total sample size of 5.6 mL is small but acknowledged. It is germane that all specimens were collected and affected in exactly the same way, and given that the primary aim was to evaluate patterns of change with secondary focus on absolute values, the advantages of this system (particularly the collection of deep and surface-before-breathing specimens on the same dive) offset any small inaccuracies introduced. Such errors are highly unlikely to have affected the fundamental conclusions of the study. In relation to these and other potential limitations, it is easy to conceive optimizations or extensions to this sort of work but often very difficult to implement them. There are substantial ethical and logistic difficulties in safely and effectively conducting field studies of this nature.
Conclusions
Predive ventilation routines used by deep free divers are effective in lowering and increasing before diving. increases markedly during a rapid free diving descent to extreme depth. It subsequently decreases precipitously during ascent, creating a significant hazard of hypoxic loss of consciousness in the latter part of ascent. This finding is confluent with those of other studies and long-standing beliefs around the pathophysiology of hypoxic loss of consciousness in free diving. increases during a rapid free diving descent to a degree unexplained by metabolic production of CO2. This probably arises as a result of compression of pulmonary gas with an increase in alveolar partial pressure of carbon dioxide ().
GRANTS
The Diving Medicine Group from the Department of Anaesthesiology at the University of Auckland receives funding through the US Navy Office of Naval Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.S., D.M., and S.J.M. conceived and designed research; T.S., H.v.W, X.C.V., D.M., P.M., and S.J.M. performed experiments; H.v.W and X.C.V. analyzed data; T.S., H.v.W, X.C.V., D.M., P.M., and S.J.M. interpreted results of experiments; H.v.W prepared figures; S.J.M. drafted manuscript; T.S., H.v.W, X.C.V., D.M., P.M., and S.J.M. edited and revised manuscript; T.S., H.v.W, X.C.V., D.M., P.M., and S.J.M. approved final version of manuscript.
ACKNOWLEDGMENTS
Stephanie Williams from the Waitemata District Health Board Point of Care Testing Laboratories loaned two i-Stat Alinity blood gas analyzers for this work and provided invaluable assistance in training team members in the use of these devices and advising on data collection. Free diving New Zealand loaned their competition free diving platform, and Brad James participated in data collection as their representative and deep safety free diver. Malcolm Bird provided a boat and acted as the shallow safety free diver. Paul Scott provided a boat and surface support activity. Jake Williams documented the operation via surface photography. The Motutere Bay TOP 10 Holiday Park allowed the use of shore facilities for logistic purposes. The Slark Hyperbaric Unit facilitated a test of syringe plunger sealing integrity at 6 atmospheres absolute pressure. The Bevin family allowed use of pool facilities for immersed tests of arterial line sampling.
REFERENCES
- 1.Lippmann J. Snorkelling and breath-hold diving fatalities in Australia, 2001 to 2013. Demographics, characteristics and chain of events. Diving Hyperb Med 49: 192–203, 2019. doi: 10.28920/dhm49.3.192-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindholm P. Loss of motor control and/or loss of consciousness during breath-hold competitions. Int J Sports Med 28: 295–299, 2007. doi: 10.1055/s-2006-924361. [DOI] [PubMed] [Google Scholar]
- 3.Lanphier EH, Rahn H. Alveolar gas exchange during breath-hold diving. J Appl Physiol (1985) 18: 471–477, 1963. doi: 10.1152/jappl.1963.18.3.471. [DOI] [PubMed] [Google Scholar]
- 4.Lindholm P, Lundgren CE. The physiology and pathophysiology of human breath-hold diving. J Appl Physiol (1985) 106: 284–292, 2009. doi: 10.1152/japplphysiol.90991.2008. [DOI] [PubMed] [Google Scholar]
- 5.Muth CM, Radermacher P, Pittner A, Steinacker J, Schabana R, Hamich S, Paulat K, Calzia E. Arterial blood gases during diving in elite apnea divers. Int J Sports Med 24: 104–107, 2003. doi: 10.1055/s-2003-38401. [DOI] [PubMed] [Google Scholar]
- 6.Bosco G, Rizzato A, Martani L, Schiavo S, Talamonti E, Garetto G, Paganini M, Camporesi EM, Moon RE. Arterial blood gas analysis in breath-hold divers at depth. Front Physiol 9: 1558, 2018. doi: 10.3389/fphys.2018.01558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosco G, Paganini M, Rizzato A, Martani L, Garetto G, Lion J, Camporesi EM, Moon RE. Arterial blood gases in divers at surface after prolonged breath-hold. Eur J Appl Physiol 120: 505–512, 2020. doi: 10.1007/s00421-019-04296-2. [DOI] [PubMed] [Google Scholar]
- 8.International Association for the Development of Apnea. AIDA competitive free diving disciplines [Online]. [https://www.aidainternational.org/Competitive] [10 February2021].
- 9.Sames C, Gorman DF, Mitchell SJ, Zhou L. Long-term changes in spirometry in occupational divers: a 10−25 year audit. Diving Hyperb Med 48: 10–16, 2018. doi: 10.28920/dhm48.1.10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loring SH, O'Donnell CR, Butler JP, Lindholm P, Jacobson F, Ferrigno M. Transpulmonary pressures and lung mechanics with glossopharyngeal insufflation and exsufflation beyond normal lung volumes in competitive breath-hold divers. J Appl Physiol (1985) 102: 841–846, 2007. doi: 10.1152/japplphysiol.00749.2006. [DOI] [PubMed] [Google Scholar]
- 11.Eger EI, Severinghaus JW. The rate of rise of PACO2 in the apneic anesthetized patient. Anesthesiology 22: 419–425, 1961. doi: 10.1097/00000542-196105000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Hesser CM. Breath holding under high pressure. In: Symposium on the Physiology of Breath-hold Diving and the Ama of Japan, edited byRahn H, Yokoyama T.. Washington DC: National Academy of Sciences (Publication 1341), 1965, p. 165–181. [Google Scholar]