Figure 1.
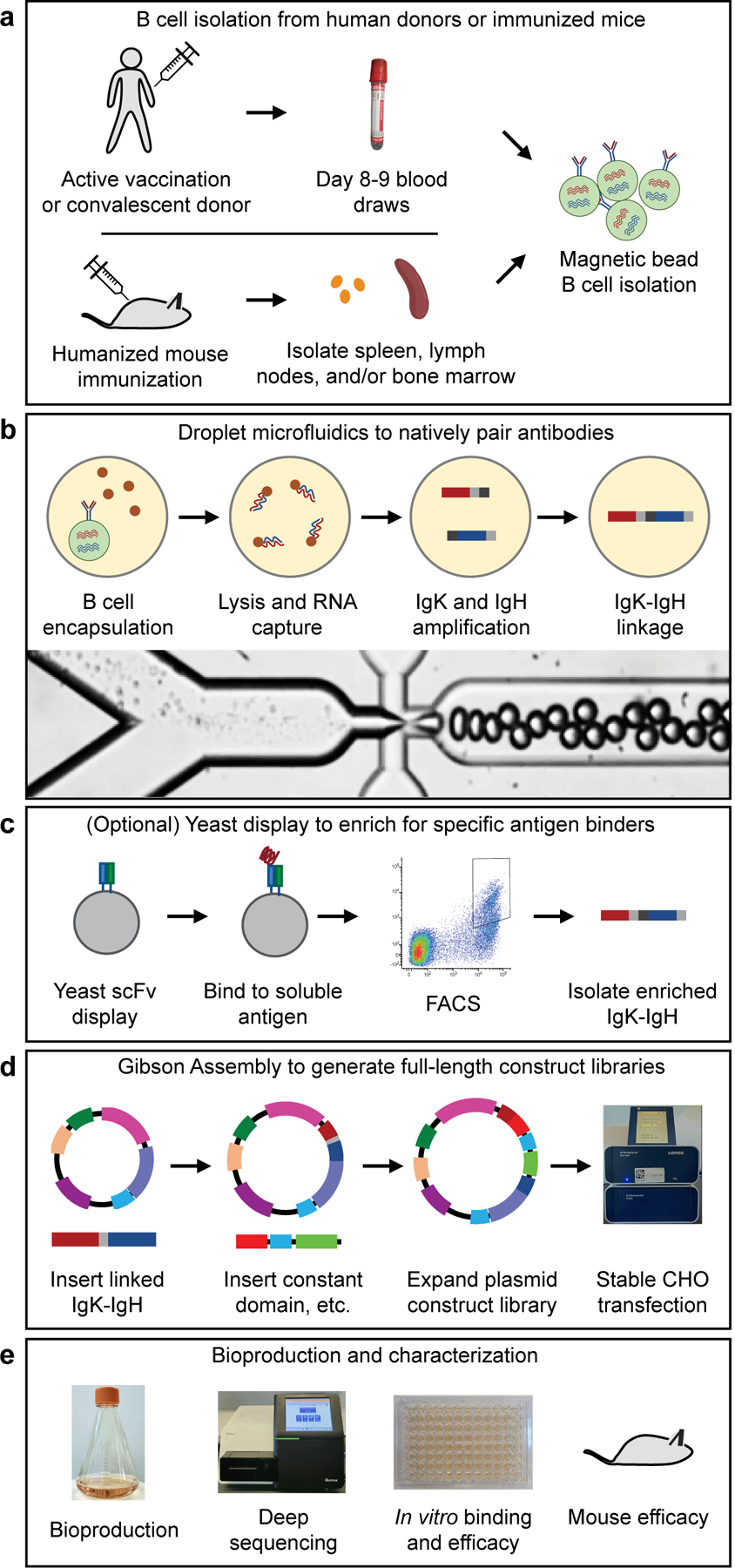
Methods used in this study for generating recombinant hyperimmune globulins. (a) B cells were isolated from human donors (vaccinated or convalescent) or immunized humanized mice. (b) Droplet microfluidics were used to capture natively paired antibody sequences from millions of single cells. (c) An optional yeast scFv display system was used to enrich for binders to a soluble antigen. (d) A two-step Gibson Assembly process converted the scFv fragment to full-length antibody expression constructs, which were then stably integrated into CHO cells following electroporation and selection. (e) After bioproduction, the libraries were characterized in many ways including deep sequencing, in vitro binding and efficacy assays, and in vivo mouse efficacy studies.
