Figure 6. Hypothesized substrate shuttles among liver, skeletal muscle and tumor that may contribute to cachexia-associated weight loss.
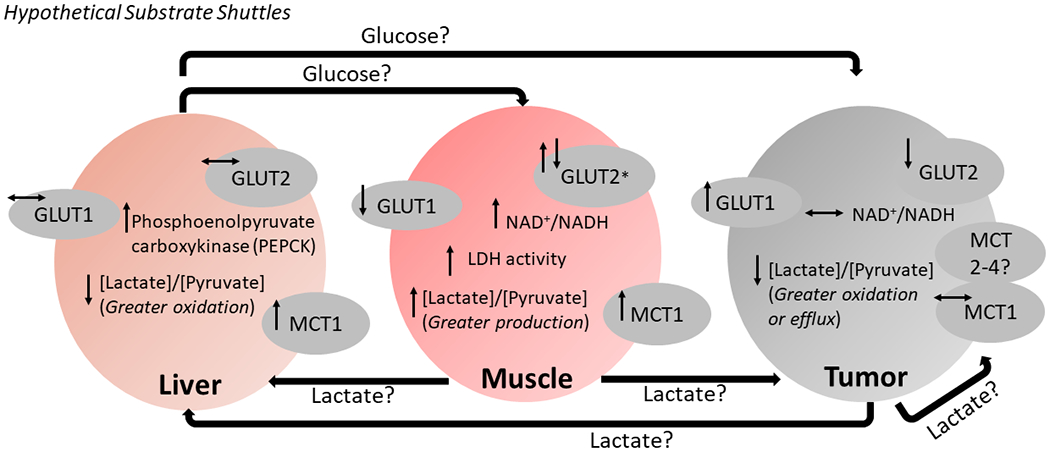
The classical Cori cycle that involves shuttling of lactate and glucose between skeletal muscle and liver appears to be active in mice with cancer cachexia. Increased lactate dehydrogenase (LDH) activity, [L-lactate]/[pyruvate], and lactate transporter MCT1 is supportive of enhanced lactate production by cachexic skeletal muscle (2 ATP yield), release into circulation and shuttling to the liver. Livers of cachexic mice had increased MCT1, suggesting lactate uptake into the liver for use in gluconeogenesis (6 ATP cost). In our prior proteome analysis, cachexic livers showed increased phosphoenolpyruvate carboxykinase (PEPCK) in this same cohort of tumor bearing mice21. PEPCK catalyzes a rate-limiting step of gluconeogenesis, therefore, increased PEPCK suggests maintained drive for hepatic gluconeogenesis. Glucose generated by gluconeogenesis can be used as energy by muscle, however decreased glucose transporter expression and ongoing accumulation of glycolytic metabolites may impair uptake and use. Another form of Cori cycle may involve lactate release from the tumor, shuttling to the liver, gluconeogenesis, and shuttling of glucose for use by the tumor. Both muscle-liver and tumor-liver shuttles would be energetically costly, with persistent shuttling possibly leading to unintended weight loss in cachexia. Another possible inter-organ substrate exchange could occur between muscle and tumor, with high production and release of muscular lactate shuttled to the energetically demanding tumor. Thus, muscle-derived lactate may serve as an important source of energy for tumors that induce subsequent cachexia. (↑, ↓) Arrows adjacent to a given metabolite or transport protein represent statistically significant comparisons for moderate and/or severe cachexia vs. weight-stable mice (PBS injected or tumor bearing). *Muscle GLUT2 initially increased in tumor-bearing weight stable and moderately cachexic mice compared to saline controls, but decreased in severe cachexia back to saline control levels.
